Low-dose multivalent conjugated vaccine composition and application
A combined vaccine and low-dose technology, applied in medical preparations containing active ingredients, bacterial antigen components, antibody medical components, etc., can solve problems such as low value, reduce the number of vaccinations, reduce production costs, and reduce missed connections rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A, C, Y, W-135 preparation of meningeal capsular polysaccharide
[0046] The strains used to produce capsular polysaccharides of Neisseria meningitides (Neisseria meningitides) group A, C, Y, W-135 were all obtained from the China Medical Culture Collection and Management Center (the strain numbers are respectively CMCC (B) 29201 , CMCC(B) 29205, CMCC(B) 29028, CMCC(B) 29037). In a 50L fermenter, the A, C, Y, W-135 groups of Neisseria meningitidis were respectively fermented and cultured. After centrifuging the obtained 30L fermentation broth, the supernatant was obtained and placed in a stainless steel tank, concentrated to 3L by ultrafiltration using a 100KD membrane bag, and 300ml of cetyltrimethyl bromide with a mass concentration of 10% was added to the concentrated solution Aqueous ammonium solution was stirred evenly and then left to stand at 4°C for 2 hours. The rested solution was centrifuged to obtain a precipitate, and sterile 1M sodium chloride aqueous sol...
Embodiment 2
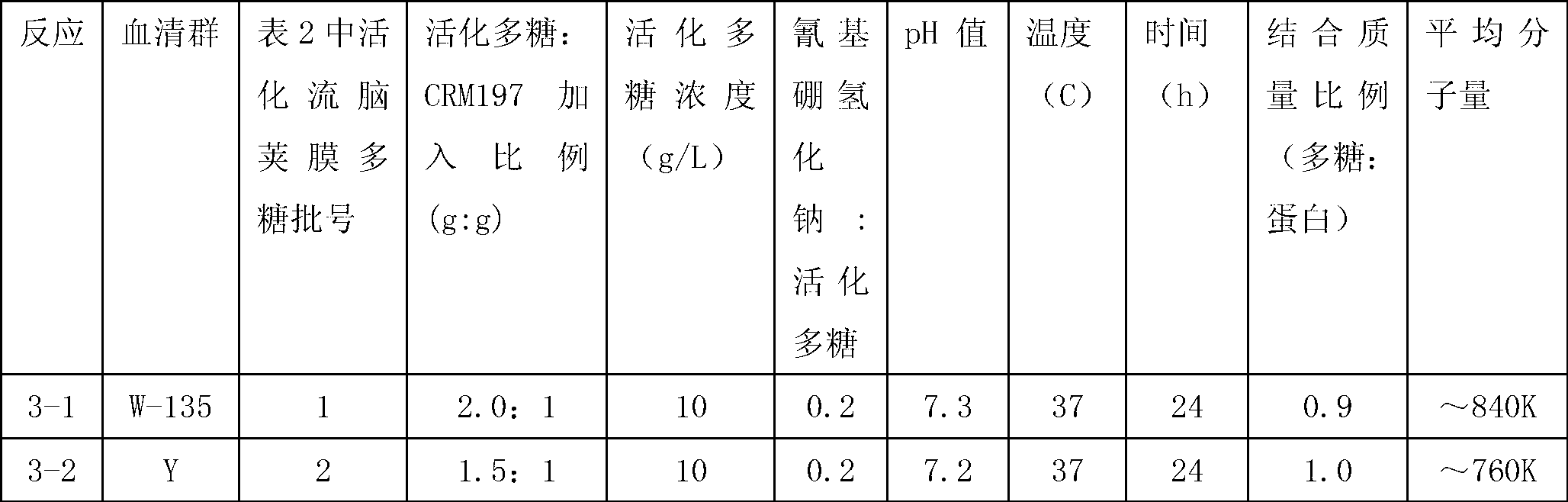
[0049] Preparation of carrier protein CRM197
[0050] The production strain Corynebacterium diphtheriae (Corynebacterium diphtheriae) was purchased from the American Type Culture Collection (ATCC), and the strain number was 39255. The lyophilized seeds producing diphtheria CRM197 protein were inoculated into test tubes containing medium and cultured for 16 hours. Transfer a portion of the culture to a 0.5-liter shaker flask containing growth medium and incubate the flask on a rotary shaker at 34.5-36.5°C for 8 hours. Transfer a portion of the culture from the flask to a 4-liter shaker flask containing growth medium Liter shaker flasks, and incubate the flasks on a rotary shaker at 34.5-36.5°C for 18 hours. The culture from this 4 liter shake flask was used to inoculate a fermenter containing 30 L of growth medium. The fermenters were incubated for 28 hours at 30-36.5°C, pH 7.4. The fermenter contents are filtered through centrifuges and depth filters into collectors.
[00...
Embodiment 3
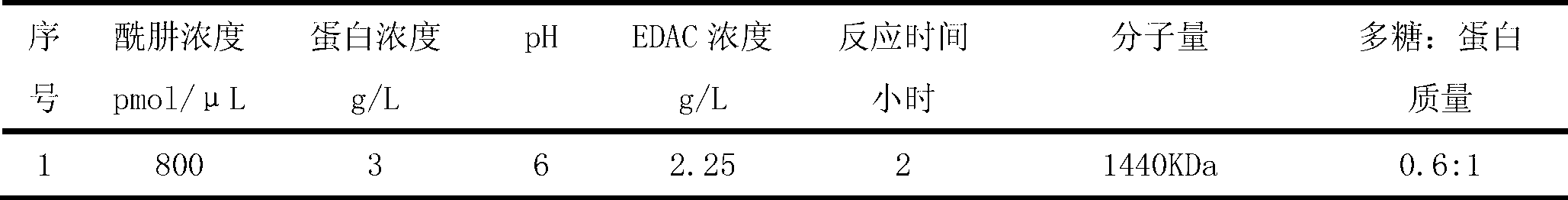
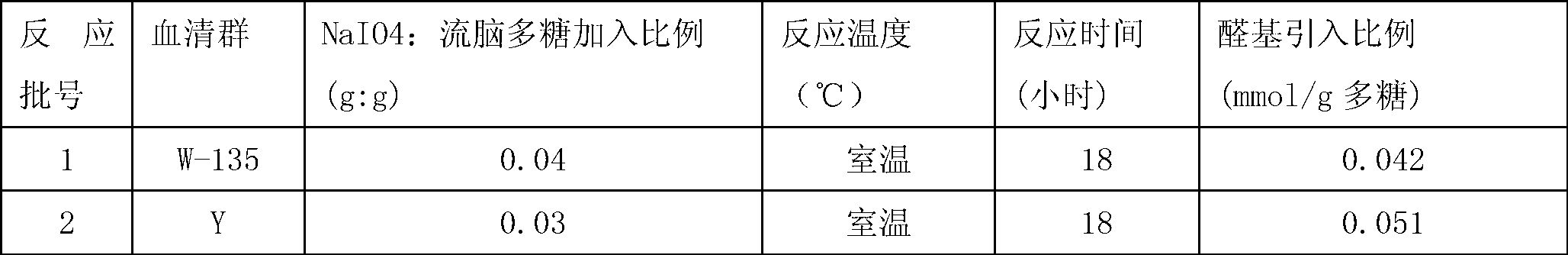
[0055] Depolymerization and Derivatization of Group A Meningococcal Capsular Polysaccharide Powder
[0056] Charge 2.5g of purified meningococcal polysaccharide powder into the reactor, add sterile sodium acetate buffer solution (50mM pH6.0) to the concentration of meningococcal polysaccharide at 4g / L at a temperature of 4°C To dissolve the meningeal capsular polysaccharide. Then the reaction tank was heated up, and some sodium acetate buffer solution (50mM pH6.0) was added to dilute the meningococcal polysaccharide to a concentration of 1.25g / L for the subsequent reaction. Heat the meningococcal polysaccharide solution to 55°C, add 30% hydrogen peroxide to a reaction concentration of 1%; keep the temperature for 2 hours, then turn off the heater and circulate the meningococcal polysaccharide solution rapidly through an ice-water bath Cool to room temperature. Use HPSEC-MALLS (high-pressure molecular sieve—MALLS system) to measure the molecular weight of the depolymerized me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com