Coumarin macromolecule polymer with anti-tumor activity and preparation method of coumarin macromolecule polymer
A technology of polymer copolymers and coumarins, which is applied in the direction of organic active ingredients, antineoplastic drugs, and medical preparations of non-active ingredients, etc., to achieve the effects of enhancing inhibition, reducing toxicity, and promoting inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
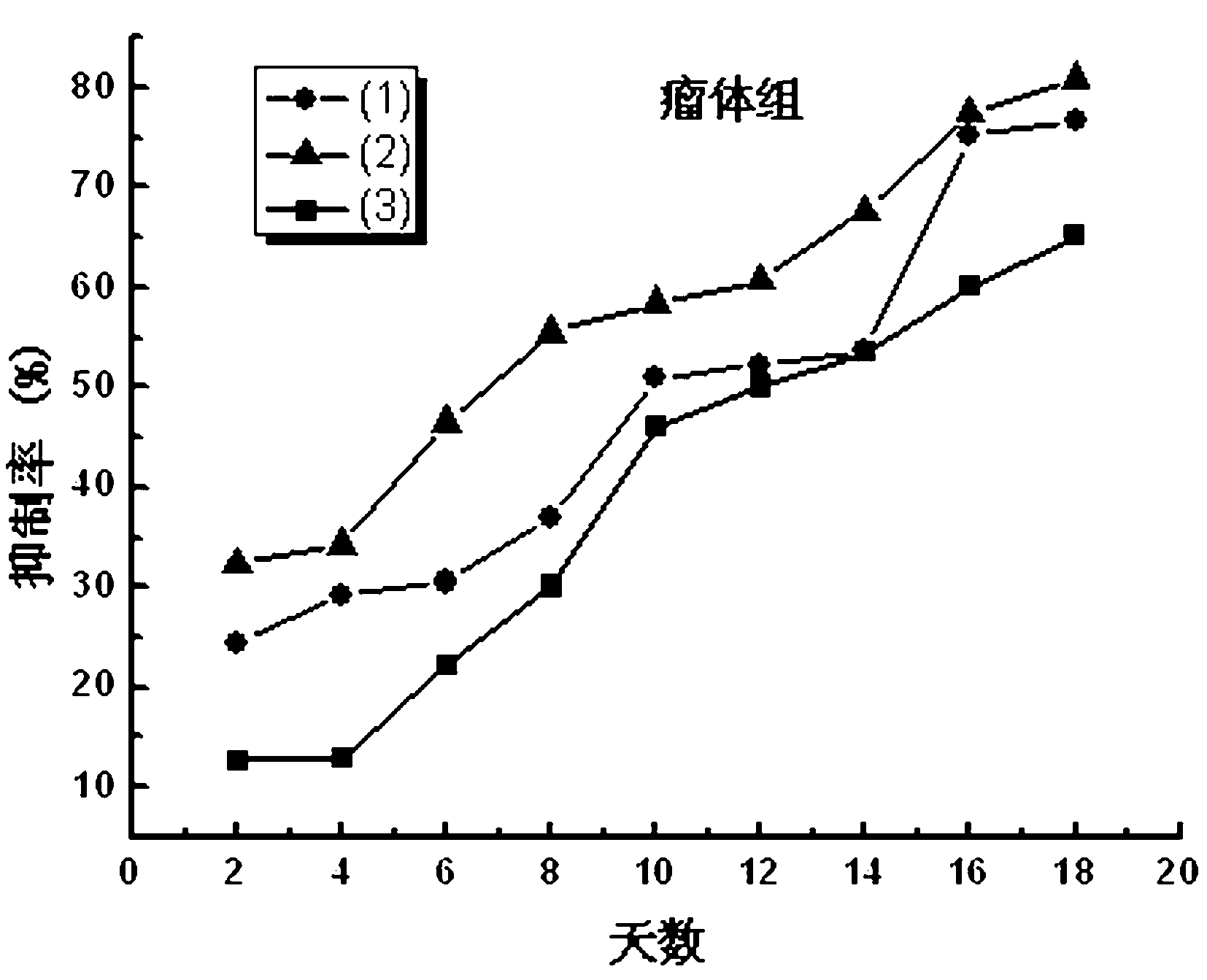
Examples
Embodiment 1
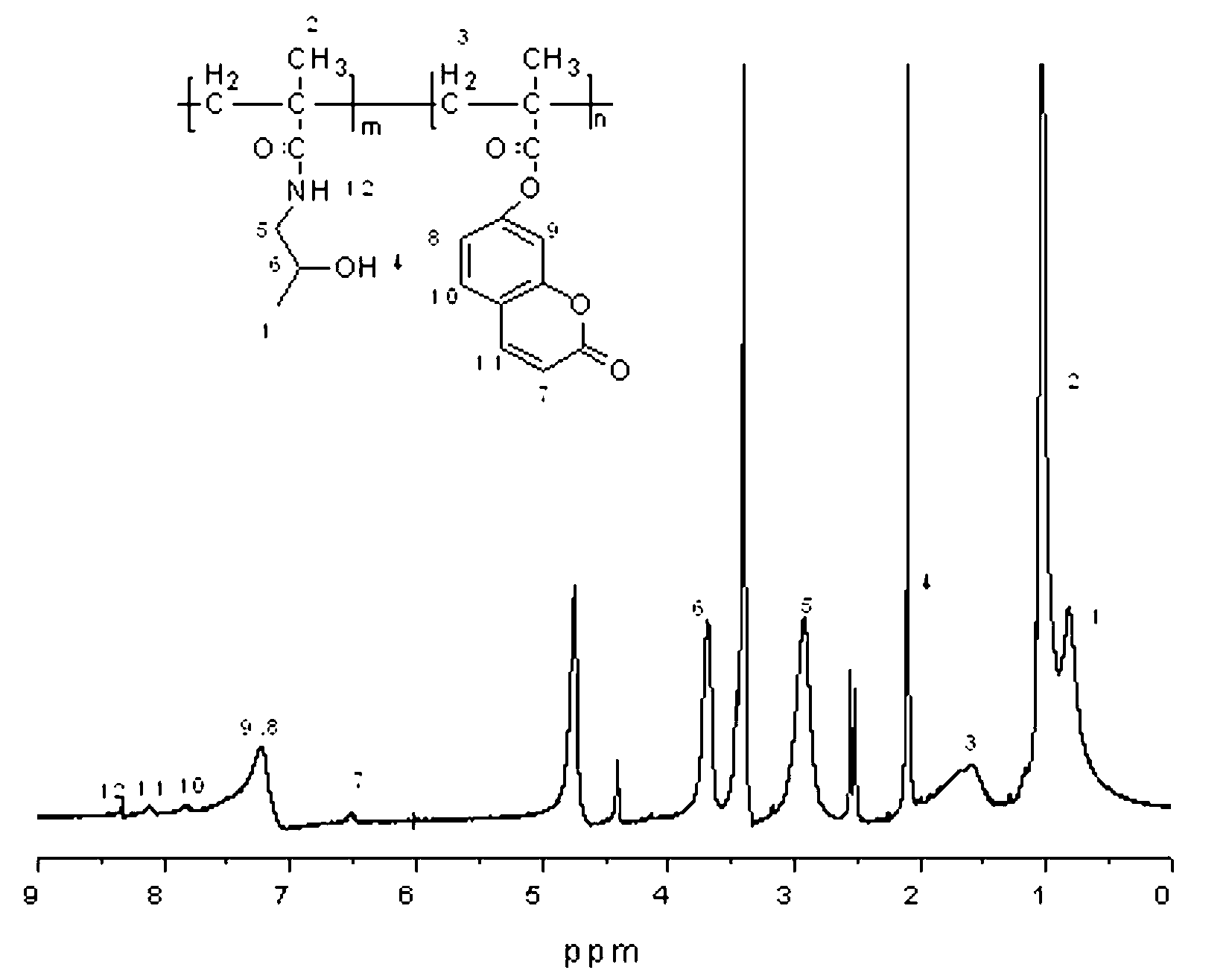
[0054] Preparation of 7-hydroxycoumarin monomer: weigh 7-hydroxycoumarin (1 mmol, 160 mg), dissolve in 20 ml of dichloromethane, cool to about 0 °C; weigh methacryloyl chloride (1.5 mmol, 150 mg), was added dropwise to a solution of 7-hydroxycoumarin in dichloromethane; weighed triethylamine (0.2 mmol, 0.02 g), added dropwise to the above solution, and then stirred overnight in an ice-water bath , to obtain a clear dark red solution; the reaction solution was evaporated on a rotary evaporator to remove the solvent to obtain a light red solid, column chromatography (ethyl acetate:petroleum ether=1:8 (v / v)) to obtain a white solid, namely For 7-hydroxy coumarin monomer. Yield 193 mg (85 %).
[0055] 1 H NMR (400 MHz, D 2 O, δ, ppm): 7.73 (d, 1H, Ar- H) ; 7.52 (d, 1H, Ar- H ); 7.27 (s, 1H, Ar- H ); 7.08-7.16 (m, 1H, Ar- H ); 6.42 (s, 1H, H -CH = C); 6.39 (s, 1H, Ar- H ); 5.83 (s, 1H, H -CH = C); 2.08 (s, 3H, -C H 3 ). 13 C NMR (400MHz, DMSO, δ, ppm): 165.07; 160...
Embodiment 2
[0059] Preparation of 7-hydroxycoumarin monomer: same as Example 1.
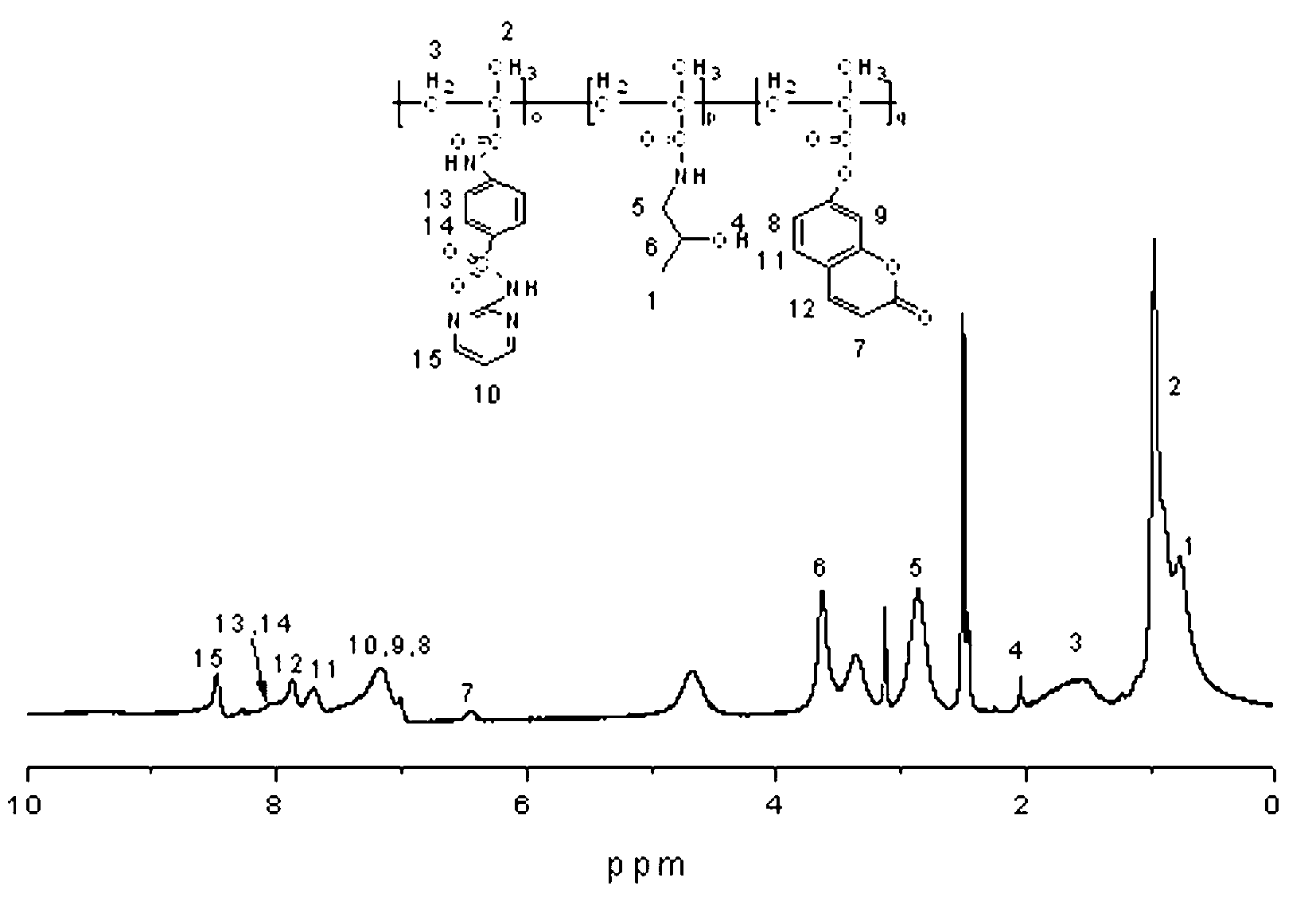
[0060] Preparation of sulfadiazine monomer: weigh sulfadiazine (1 mmol, 250 mg), dissolve in a mixed solution of acetone and sodium hydroxide (1:1.5 (v / v)), cool to about 0°C; Acid chloride (1.5 mmol, 150 mg) was added dropwise to the above cooled solution, stirred overnight in an ice-salt bath, and a yellow precipitate was obtained; after suction filtration and drying, use methanol-water mixed solvent (2:0.5 (v / v) ) recrystallized, then suction filtered, and dried in a vacuum oven at 40 °C for 72 hours to obtain a light yellow solid which is the sulfadiazine monomer (B). Yield was (230 mg, 72%).
[0061] 1 H NMR (400 MHz, DMSO, δ, ppm ): 11.69 (s, 1H, -CO-N H -), 10.14 (s, 1H, -SO 2- N H -), 8.48-8.49 (d, 2H, pyrimidine- H ), 7.92 (q, 4H, Ar- H ), 7.0 (s, 1H, pyrimidine- H ), 5.82 (s, 1H, H-CH = C), 5.56 (s, 1H, H -CH = C), 1.92 (s, 3H, CH 2 = C-C H 3 ). 13 C NMR (400 MHz, DMSO, δ, ppm): 167....
Embodiment 3
[0065] Preparation of 7-hydroxycoumarin monomer: same as Example 1.
[0066] Preparation of coumarin-based polymer copolymer (1): Weigh 7-hydroxycoumarin monomer (8%, 23 mg, 0.1 mmol), HPMA (92%, 165 mg, 1.15 mmol) in a reaction flask , add 0.5ml of DMSO, stir to make it completely dissolved, then add 1ml of acetone and stir evenly; then add azobisisobutyronitrile (8%, wt, 15 mg,); vacuumize and fill with nitrogen, at 60℃ Polymerized for 24 hours. Dissolve the polymerization reaction solution with acetone precipitation methanol (2:1 (v / v)) for 3 times and then filter the precipitate to obtain a white solid. Centrifuge to remove small molecules, and dry the liquid after centrifugation to obtain a white solid that is a polymer drug (1). The yield was (138 mg, 73 %).
[0067] 1 H NMR (400 MHz, DMSO, δ, ppm): 8.10 (1H, Ar- H of UMB), 7.88 (1H, Ar- H of UMB), 7.25 (1H. Ar- H of UMB), 7.21 (1H, Ar- H of UMB), 6.53 (1H, Ar- H of UMB), 3.71 (1H, CH 3 C H (OH)CH 2 NH- ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com