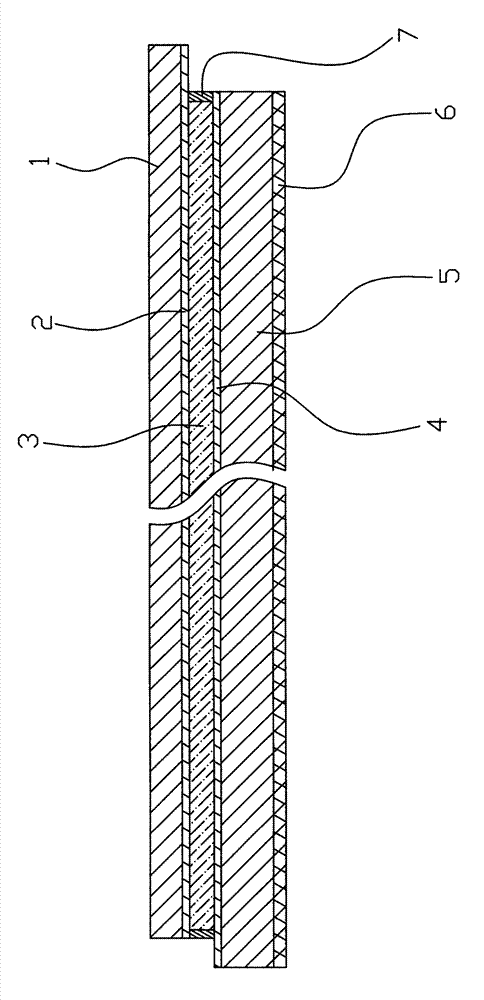
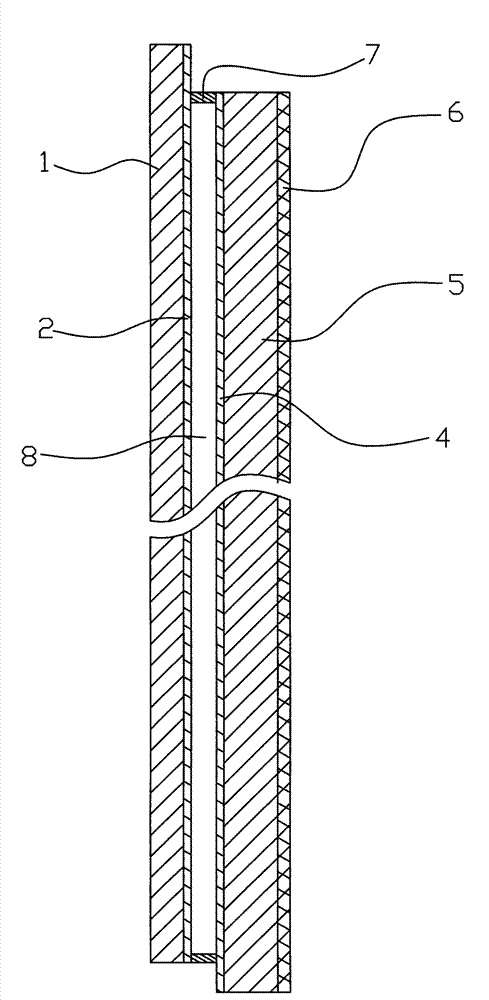
Electrochromic material and electrochromic device
An electrochromic material and electrochromic device technology, applied in the direction of color-changing fluorescent materials, instruments, chemical instruments and methods, etc., can solve the problems of high production process requirements, high cost, large limitations, etc., and increase the electrical conductivity. , Fast fading rate, and the effect of improving the discoloration rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
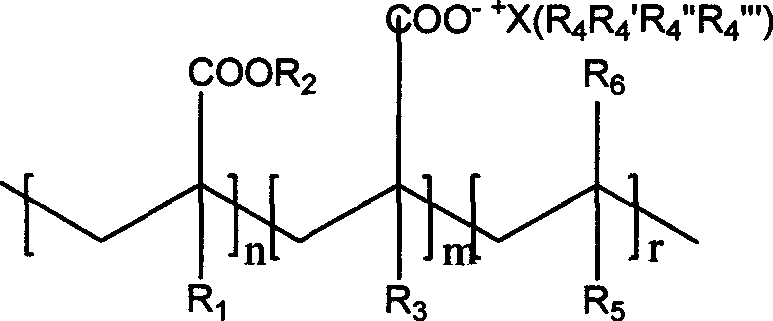
[0042]The preparation method of the acrylic acid ionic salt polyelectrolyte comprises the following steps: the step of synthesizing the acrylic acid monomer of the ionic salt; the acrylic acid monomer of the synthesized ionic salt is copolymerized with the acrylic monomer and the olefin monomer to prepare an ionic polymer electrolyte A step of. The specific steps of the acrylic acid monomer for the synthesis of ionic salts are as follows: dissolve 0.01-0.012 mol of quaternary ammonium salt or quaternary phosphonium salt in 10-100 ml of absolute ethanol, add 0.01-0.012 mol of acrylic acid monomer compound, Then add 0.01-0.012 mol of potassium hydroxide or sodium hydroxide solid particles, at room temperature until the reaction is complete, distill the resulting filtrate at 30-50°C to remove the absolute ethanol solvent, and then place it at -10-0 Cool and crystallize at ℃ for 1 to 10 hours, filter the cooled mixture under reduced pressure to obtain acrylic acid ion salt monomer...
Embodiment 1
[0050] Example 1, take 0.01mol of tetraethylammonium bromide and dissolve in 20ml of absolute ethanol, add 0.01mol of acrylic acid, then add 0.01mol of sodium hydroxide solid particles, react at room temperature for 24 hours and then filter. The resulting filtrate was distilled off under reduced pressure at 45°C to remove the absolute ethanol solvent, and placed at -10°C for cooling and crystallization for 2 hours. The cooled mixture was filtered under reduced pressure to obtain tetraethylammonium acrylate solid particles. Dissolve 1mol of methyl methacrylate, 0.02mol of tetraethylammonium acrylate and 0.5mol of styrene in 1000ml of toluene solvent, add 0.001mol of azobisisobutyronitrile as the initiator of free radical polymerization, and stir the reaction at 80°C 12 hours. The obtained mixture is added dropwise into petroleum ether to precipitate the acrylic acid ionic salt polyelectrolyte to obtain the desired new acrylic acid ionic salt polyelectrolyte. The chemical stru...
Embodiment 2
[0053] Example 2, 0.01 mol of tetrabutylphosphonium bromide was dissolved in 10 ml of anhydrous methanol, 0.01 mol of methacrylic acid was added, and then 0.01 mol of potassium hydroxide solid particles were added, reacted at room temperature for 24 hours and then filtered. The resulting filtrate was distilled off under reduced pressure at 35°C to remove the anhydrous methyl ethanol solvent, and placed at -5°C for cooling and crystallization for 8 hours. The cooled mixture was filtered under reduced pressure to obtain solid particles of tetrabutylphosphonium methacrylate. 0.5mol butyl methacrylate, 0.05mol tetrabutylphosphonium methacrylate and 1mol methyl methacrylate were dissolved in 500ml ethylene carbonate solvent, and 0.002mol of azobisisobutyronitrile was added as the initiator of free radical polymerization. The reaction was stirred at 60°C for 12 hours. The obtained mixture is added dropwise into petroleum ether to precipitate the acrylic acid ionic salt polyelectrol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com