Compound and nucleic acid complex molecule and nucleic acid complex and preparation method and application thereof
A compound molecule and compound technology, applied in the field of preparation of small interfering nucleic acid drugs, can solve the problems of small specific gravity, toxicity and other safety indicators that cannot be fully trusted, and achieve the goal of reducing toxicity, reducing dosage, and improving tissue specificity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0039] According to a preferred embodiment of the present invention, the compound has a structure represented by formula V,
[0040] Formula V,
[0041] Wherein, B is selected from N, P=O, C-X 5 -A 5 or C-R 8 ;A 1 、A 2 、A 3 、A 4 and A 5 Each independently is a group shown in formula I or a group shown in formula II; X 1 、X 2 、X 3 、X 4 and X 5 Each independently is an optionally substituted C1-C20 alkylene group or a group represented by formula III;
[0042] Formula III,
[0043] Among them, R 6 and R 37 is a bond or an optionally substituted C1-C14 alkylene group;
[0044] R 7 is an optionally substituted C1-C20 hydrocarbyl or targeting group; R 8 is H, an optionally substituted C1-C20 hydrocarbon group or a targeting group.
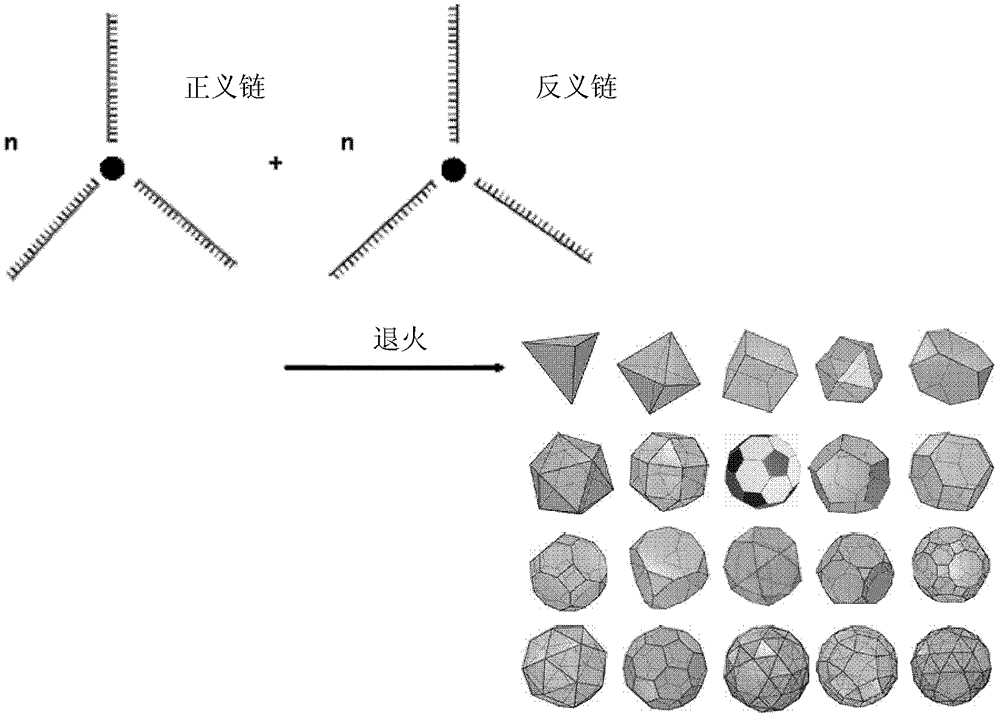
[0045] In the present invention, the three or more first connection structures are the basis for the compound to be used as a carrier to form a spatial topology, and the three or more are preferably 3-6, such as 3, 4, 5 or 6 ...
Embodiment 1
[0225] This example is used to illustrate the synthesis of the central molecule
[0226] (1) Compound (2Z, 2′Z, 2″Z)-4,4′, 4″-(2,2′, 2″-nitrilo three (ethane-2,1 Synthesis of -diyl)tri(azanediyl))tri(4-carbonylbut-2-enoic acid)
[0227]
[0228] Add compound (1) (13.38mmol, 2.016g) into a dry 500mL round bottom flask, then add 200mL of anhydrous dichloromethane, stir evenly with electromagnetic. Add maleic anhydride (45.16mmol, 4.435g) into the round bottom flask, increase the stirring speed, and heat to reflux at 50°C for 2h. The solvent dichloromethane was removed by rotary evaporation to obtain 4.984 g of compound (4) as a yellow solid with a yield of 83%.
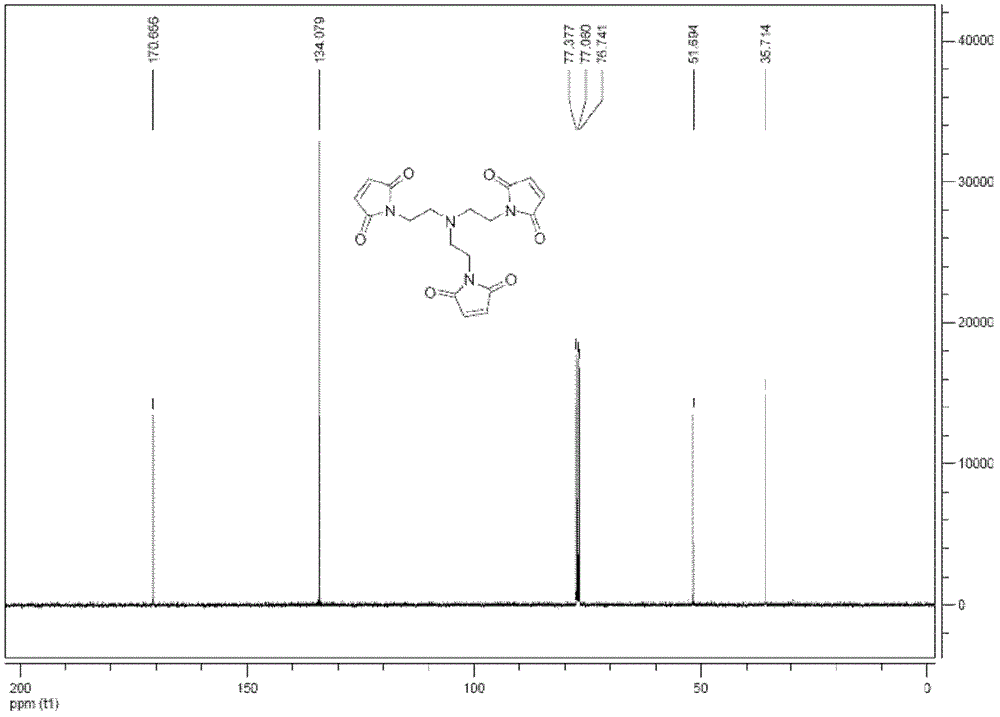
[0229] (2) Compound 1,1',1"-(2,2',2"-nitrilotri(ethane-2,1-diyl))tri(1H-pyrrole-2 , 5-diketone) synthesis
[0230]
[0231] Add compound (4) (5mmol, 2.204g), triethylamine (4.158mmol, 420mg), sodium acetate (4.085mmol, 335mg) respectively into a dry 100mL round bottom flask, then add 10mL of acetone, and stir...
Embodiment 2
[0234] This embodiment is used to illustrate the preparation of the nucleic acid complex molecule N1 of the present invention
[0235] Dissolve the terminal sulfhydryl-modified oligonucleotide R1 in 100 microliters of RNase-free water, add Tri-HCl buffer (pH=8.0, final concentration is 20mM), the final concentration of R1 is 1mM, and mix well; 0.11 mM compound (5), placed in a flowing high-purity argon atmosphere for 5 min, and incubated at 45° C. for 2 h. Repeat the above steps of adding compound (5) and argon protection and reacting for 2 h twice. Afterwards, polyacrylamide (6% urea) electrophoresis and gel purification were carried out, and a single band corresponding to the position of the 90nt natural oligonucleotide was cut out for gel elution. After 2 hours, it was washed with 0.3M sodium acetate and 75% ethanol precipitation. Dehydration for 1h. Centrifuge at 13000 rpm for 15 min at 4°C, discard the supernatant to obtain N1.
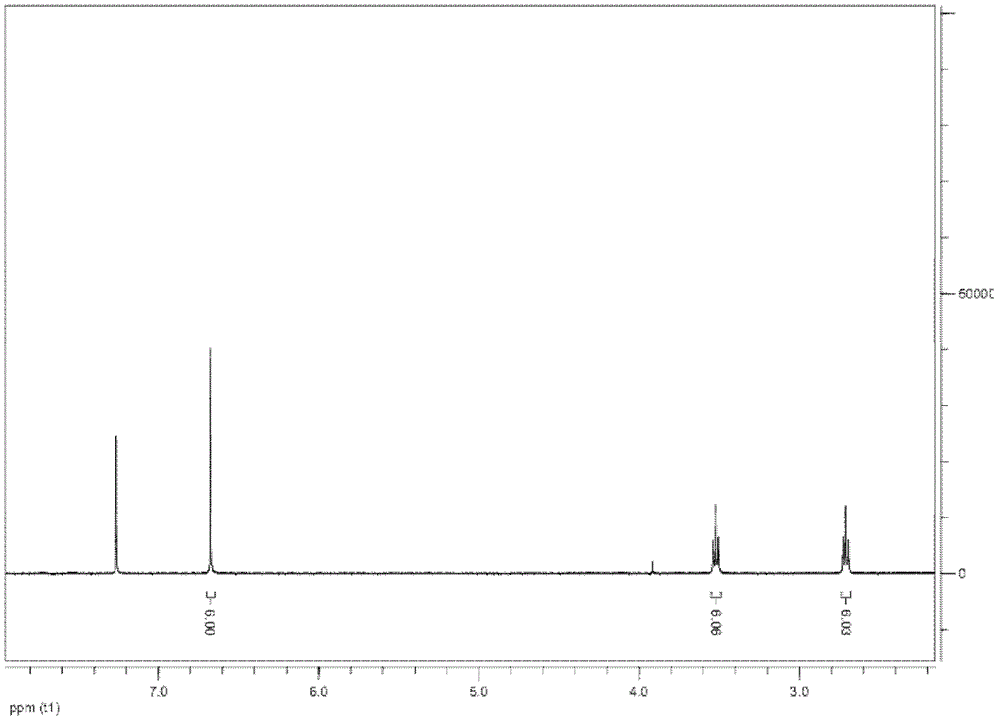
[0236] N1 was detected by 6% and 15% po...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com