Triterpene derivative and preparation method and application
A use and compound technology, applied in the field of triterpenes and their derivatives, can solve the problems that the biological activity of triterpenoids has not been reported, and prevent the spread of HIV virus, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
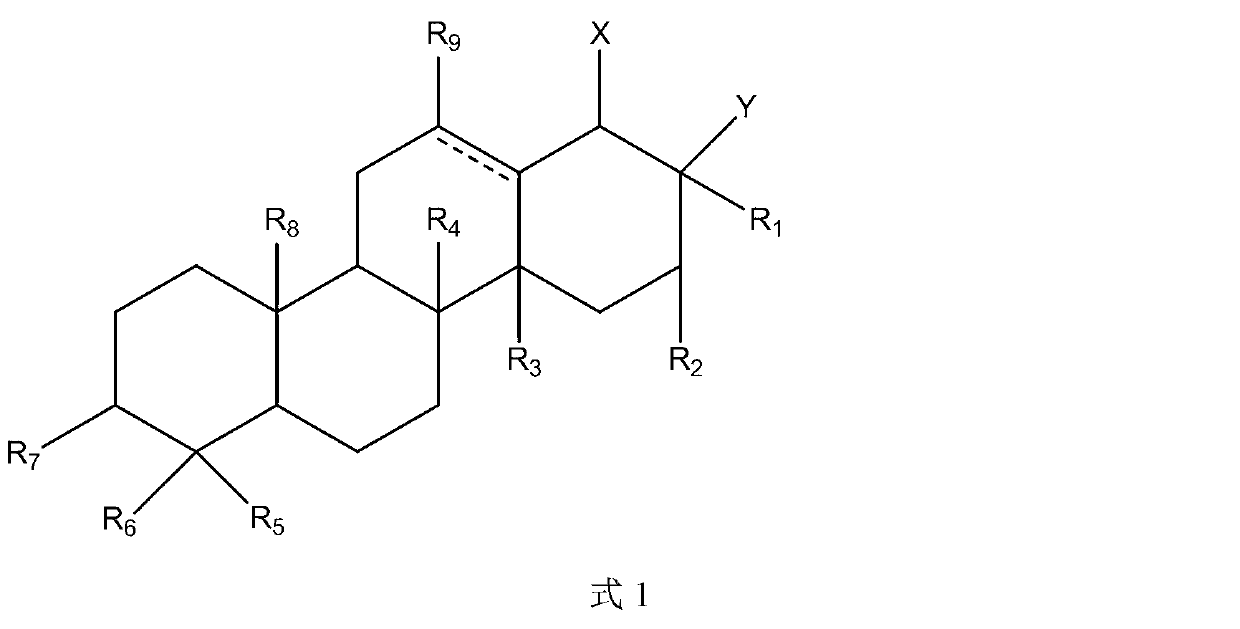
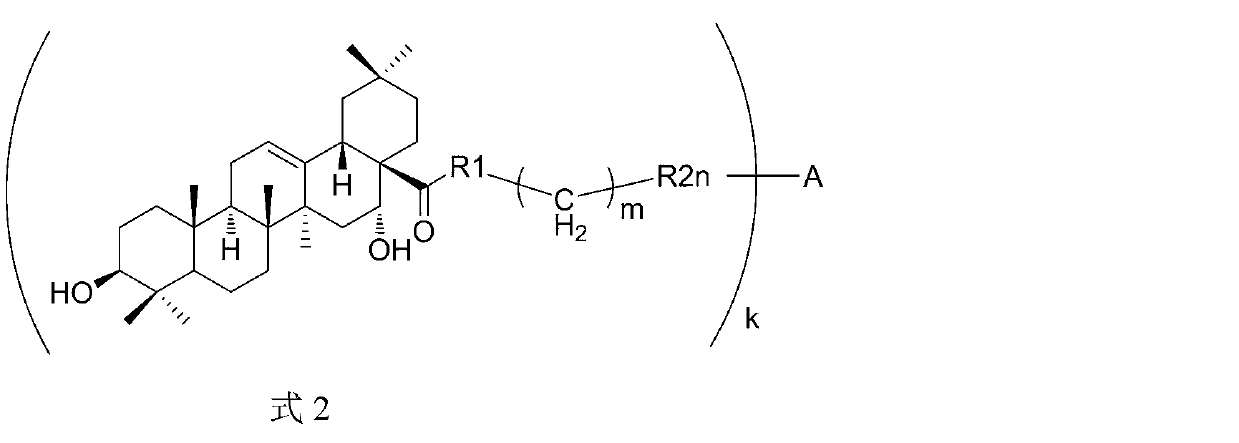
[0072] The preparation method of the compound of the present invention
[0073] Another aspect of the present invention provides a method for preparing the above compound.
[0074] The triterpene compounds and derivatives involved in the present invention can be extracted from natural plants, and / or chemically synthesized, semi-synthesized or structurally modified. In one embodiment of the invention, some triterpenoids can be obtained by extracting from plants or purchased from the market, and some other triterpenes can be obtained by structural modification or chemical synthesis or semi-synthesis of the above-mentioned triterpenoids.
[0075] The extraction method includes immersing triterpenoid-rich plants in a polar solvent to reflux, filtering to remove insoluble matter, concentrating, acid treatment, and finally separating the triterpenoids by silica gel column chromatography (e.g. dichloromethane / methanol gradient elution). Terpene aglycone. Those skilled in the art ha...
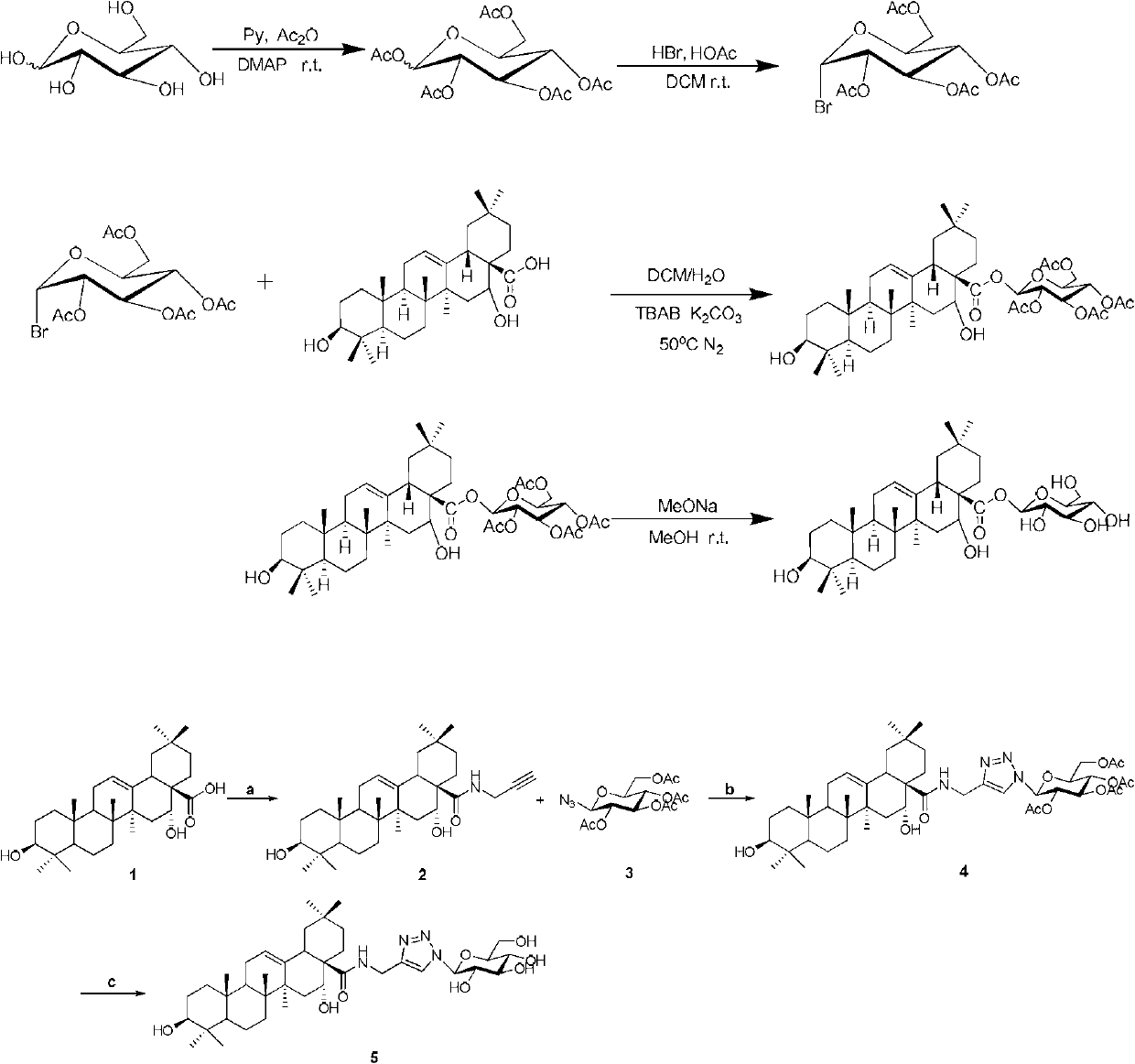
Embodiment 1
[0147] Example 1 Synthesis of echinocytic acid-glucose derivatives
[0148]
[0149] Take 3g of D-glucose in a 50mL reaction bottle, add 24mL of pyridine to dissolve it, add 12mL of acetic anhydride in turn, and a catalytic amount of DMAP, and stop the reaction after overnight reaction at room temperature. TLC monitoring, developer PE:EA=1:1. After the solvent was spin-dried, 20mL of the developing agent (PE:EA=1:1) was dissolved and separated by a flash column for use.
[0150] Take 390mg of the above product in a 25mL reaction bottle, dissolve in 3mL DCM, slowly add 0.21mL of HBr-HOAc solution dropwise under ice bath, react for 1h, place it at room temperature for reaction, monitor by TLC, developer PE:EA=2:1. After 12 hours of reaction, the reaction was stopped. After adding 20mL DCM to dilute, successively add 20mL distilled water, 20mL saturated NaHCO 3 Solution washing, combined organic layers, MgSO 4 After drying, column separation and purification, the developme...
Embodiment 2
[0156] Example 2 Synthesis of echinocytic acid-glucose derivatives by click reaction
[0157] Reagents and reaction conditions: (a) TBTU, DIEA, prop-2-yn-1-amine, THF, rt; (b) CuSO 4 ,sodium L-ascorbate,CH 2 Cl 2 -H 2 O, rt; (c) 4NaOH, MeOH, rt.
[0158] operate:
[0159] (a) Dissolve 472mg (1mmol) of EA in 10mL of THF, add 385mg (1.2mmol) of TBTU and 0.4mL (2.4mmol) of DIEA, stir the reaction at room temperature for 3h until the reaction of EA is complete, then add 0.13mL of propargylamine into the system (2mmol), continue to react for 0.5h until the reaction is complete. The precipitate was filtered off, the filtrate was concentrated under reduced pressure, the sample was mixed with silica gel column chromatography, petroleum ether: ethyl acetate = 2:1, and 458 mg of white solid product 2 was obtained, with a yield of 90%. 1 H NMR (400MHz, CDCl 3 ):δ0.79,0.81,0.91,0.93,0.95,1.00,1.38(s,7×CH 3 ),0.73-2.30(m,other aliphatic ring protons),2.21(t,1H,J=2.6Hz),2.70(dd,1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com