Production process for producing antiviral medicament ribavirin through bacillus amyloliquefaciens precursor addition fermentation method
A technology for the production of amylolytic spores and production technology, which is applied in the field of the production of antiviral drug ribavirin by adding precursors to the fermentation method of genetically engineered bacteria, which can solve the problems of many by-products, high reaction temperature, and high cost, and achieve short fermentation cycle , easy to operate, maintain the effect of activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
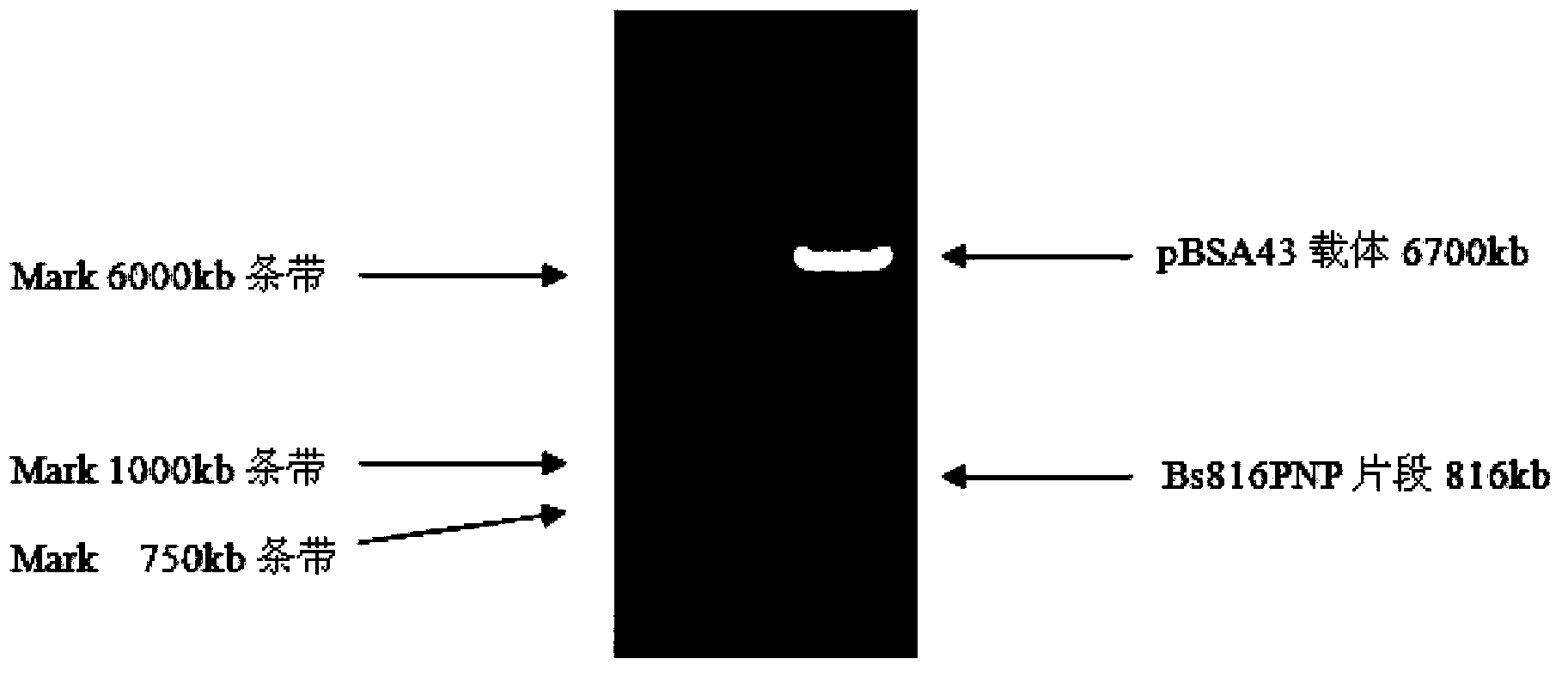
[0025] Example 1 Construction of genetically engineered bacteria RBVM (pBSA43-Bs816PNP)
[0026] The carrier pBE2 was modified by relevant molecular biology techniques and named pBSA43, which has the following characteristics: ① Equipped with a P43 promoter that can be recognized by Bacillus amyloliquefaciens RNA polymerase, ensuring that the target gene can be efficiently expressed in the host bacteria, And there is no need for chemical inducers IPTG or lactose induction. ②According to the characteristics of the Bacillus amyloliquefaciens restriction-modification system, the BamH I restriction site on the plasmid was removed to make the plasmid easier to transform; ③The sequence encoding the signal peptide in the sacB gene sequence can be fused to the 5 ' end, allowing the gene of interest to be secreted into the medium.
[0027] Construction of pBSA43 plasmid: Using PCR technology, the sequence of the P43 promoter and the sequence encoding the signal peptide in the sacB gen...
Embodiment 2
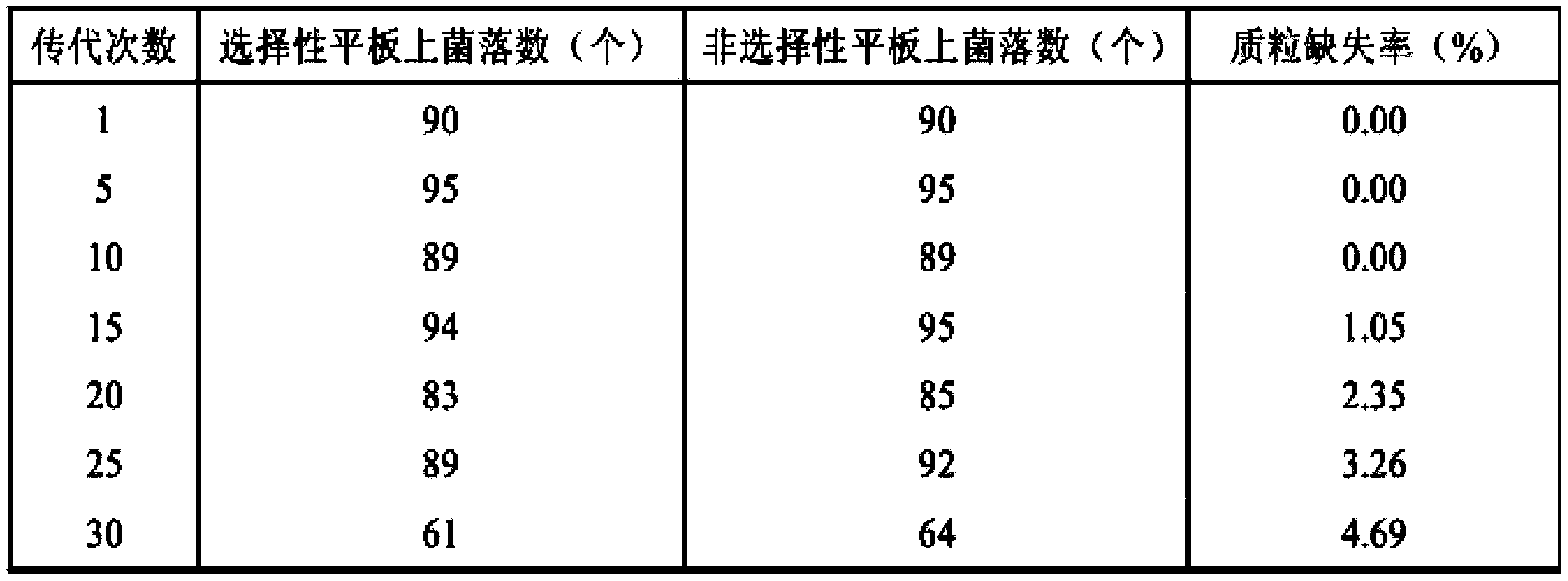
[0030] Example 2 Engineering bacteria RBVM (pBSA43-Bs816PNP) plasmid stability
[0031] The plate dilution counting method was used. The engineered bacteria (constructed in Example 1) frozen in a glycerol tube at -80°C were streaked in three sections on an LB plate containing 10 μg / L kanamycin, cultured at 37°C for 16 hours, and a single colony was picked and inoculated in 5ml of liquid Selective medium (LB / Kmr ), after 12 hours of shaking tube culture, transfer 1% of the inoculum into 5ml liquid non-selective medium (LB), and start the continuous shaking tube culture test. Transplant once every 12 hours, the inoculum size was 1%, continuous passage for 30 times, every 5 times sampling was carried out for plate dilution to detect the degree of loss of the plasmid. During the detection, take 1ml of the bacterial solution and dilute it step by step with sterile water, and get three suitable gradients. This experiment selects 10 -7 、10 -8 、10 -9 Three gradients were plated on...
Embodiment 3
[0036] Example 3 Fermentation of engineered bacteria RBVM (pBSA43-Bs816PNP) to produce ribavirin in shake flasks
[0037] Pick a ring of engineering bacteria RBVM (pBSA43-Bs816PNP) lawn constructed in Example 1 from the freshly activated slant and put it in the seed medium (glucose 2%, beef extract 1%, (NH 3 ) 2 SO 4 1%, yeast powder 0.75%, MgSO 4 ·7H 2 O 0.1%, KH 2 PO 4 0.1%, corn steep liquor 0.2%, soybean concentration 0.2%, pH7.0~7.2), 500mL triangular bottle with 25mL liquid volume, 9 layers of gauze seal. Place on a rotary shaker at 35°C for 10 h with shaking at a speed of 220 r / min.
[0038] Insert 5% inoculum into the fermentation medium (8% glucose, 0.8% yeast extract, 1.6% bean concentration, 1% corn steep liquor, (NH 3 ) 2 SO 4 1%, MgSO 4 ·7H 2 O 0.1%, KH 2 PO 4 0.1%, FeSO 4 2mg / L, pH7.3~7.4). A 500mL Erlenmeyer flask was filled with 30mL liquid, cultured with shaking at 33°C for 60h, and the rotation speed was 220r / min. Use 25% ammonia water to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com