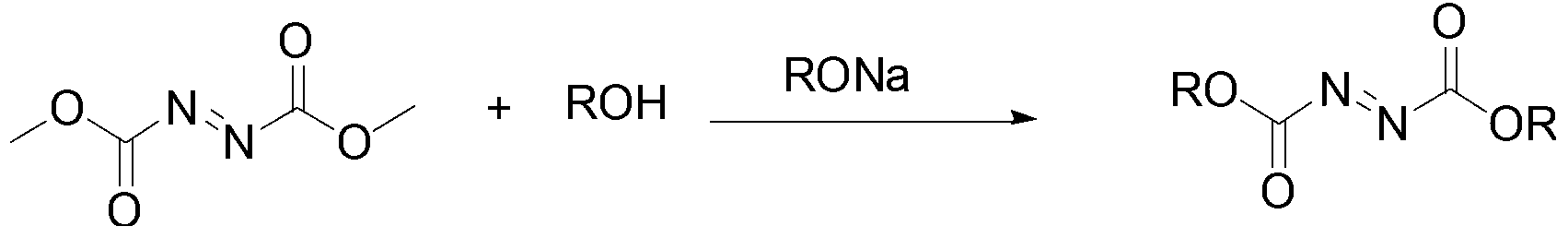
Esterification method for preparing azodicarbonic acid
A technology of dialkyl azodicarboxylate and diethyl azodicarboxylate, which is applied in the field of dialkyl azodicarboxylate represented by the formula ROOCN=NCOOR, can solve non-compliance, high price and environmental pollution problems such as mild reaction conditions, low production costs, and short process routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation of embodiment 1 diisopropyl azodicarboxylate
[0031] In a 250mL three-necked round-bottomed flask equipped with a magnetic stirrer, a 25mL constant pressure dropping funnel and a rectification device, add 100mL of isopropanol, and under nitrogen protection, add 0.46g (0.02mol) of sodium metal, and wait for the sodium metal to After complete dissolution, 14.60 g of dimethyl azodicarboxylate was added dropwise and reacted for 24 hours under reflux until no methanol evaporated to stop the reaction. The reaction solution was neutralized to pH 7 with 10% hydrochloric acid (mass fraction, the same below), and the organic phase was extracted three times with dichloromethane. The organic phases were combined, dried overnight (10 h) over anhydrous sodium sulfate, and unreacted Isopropanol was recovered and distilled under reduced pressure to obtain 16.52 g of wine-red diisopropyl azodicarboxylate (81.8% yield).
[0032] 1 HNMR (CDCl 3 ,300MHz)δ(ppm):5.33-5.43...
Embodiment 2
[0034] The preparation of embodiment 2 diisoamyl azodicarboxylate
[0035]In a 250mL three-necked round-bottomed flask equipped with a magnetic stirrer, a 25mL constant pressure dropping funnel and a rectification device, add 100mL of isoamyl alcohol, and under nitrogen protection, add 0.46g (0.02mol) of sodium metal, and wait for the sodium metal to After complete dissolution, 14.60 g of dimethyl azodicarboxylate was added dropwise, and the reaction was carried out under reflux for 16 hours until no methanol evaporated to stop the reaction. The reaction solution was neutralized with 10% hydrochloric acid solution to pH 7, the organic phase was extracted three times with dichloromethane, the organic phase was combined, dried overnight (10 h) over anhydrous sodium sulfate, unreacted isobutanol was evaporated to recover, and the Distilled under pressure to obtain 21.91 g of orange-red diisoamyl azodicarboxylate (85.2% yield).
[0036] 1 HNMR (CDCl 3 ,300MHz)δ(ppm):4.13(4H,t,C...
Embodiment 3
[0039] The preparation of embodiment 3 dicyclohexyl azodicarboxylate
[0040] In a 250mL three-necked round-bottomed flask equipped with a magnetic stirrer, a 25mL constant-pressure dropping funnel and a rectification device, add 100mL of cyclohexanol, and under nitrogen protection, add 0.46g (0.02mol) of sodium metal, and wait for the sodium metal to After complete dissolution, 14.60 g of dimethyl azodicarboxylate was added dropwise, and the reaction was carried out under reflux for 12 hours until no methanol evaporated to stop the reaction. The reaction solution was neutralized with 10% hydrochloric acid solution to a pH value of 6, the organic phase was extracted three times with dichloromethane, the organic phase was combined, dried over anhydrous sodium sulfate (8h), unreacted cyclohexanol was distilled off and recovered, and Ethyl acetate-petroleum ether mixture (the volume ratio of the two is 1:5) was recrystallized to obtain 22.63 g of dicyclohexyl azodicarboxylate as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com