Nano-lithium iron phosphate material suitable for high rate power battery and preparation method thereof
A lithium iron phosphate and power battery technology, applied in the field of nano-lithium iron phosphate materials, can solve problems such as low electronic conductivity and ionic conductivity, reduced battery capacity, and poor material rate characteristics, and achieve improved conductivity and electrical performance. , The effect of high product controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Put ferrous oxalate (1177g), lithium carbonate (263g), ferric dihydrogen ammonium phosphate (1250g), glucose (500g), isopropanol (6000g) in a ball mill for 3 hours, and then ultrafine in a sand mill Grind for 3 hours, then dry in a double-cone vacuum dryer for 4 hours to obtain the precursor. The D50 of the precursor is 0.4~0.8um; Melt and burn at 700°C for 20 hours, cool to room temperature at a cooling rate of 4°C / min to obtain lithium iron phosphate; then pass through a jet mill for 10 minutes, and finally mix with a double-helix conical mixer for 2 hours to obtain iron phosphate Lithium products.
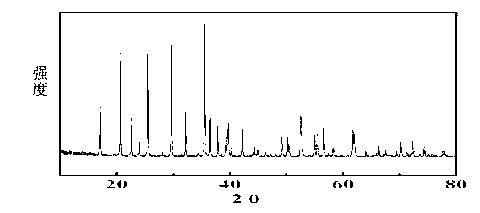
[0035] Such as figure 1 As shown, the X-ray diffraction analysis results show that the prepared lithium iron phosphate is olivine type lithium iron phosphate;
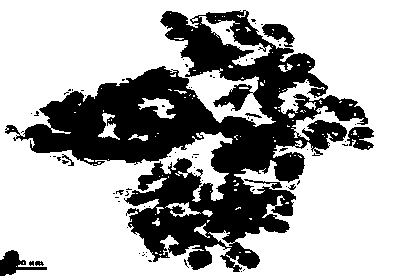
[0036] figure 2 Scanning electron microscope photos show that the primary particles are between 50~100nm, the particles are spherical, and the aspect ratio is 1.3~1. image 3 Transmission electron microscope ...
Embodiment 2
[0041] Put ferrous oxalate (1798g), lithium dihydrogen phosphate (1046g), glucose (400g), and isopropanol (6000g) in a ball mill for 3 hours, then grind them ultrafinely in a sand mill for 3 hours, and then grind them in a double Dry in a cone vacuum dryer for 4 hours to obtain the precursor, the precursor D50 is 0.4~0.8um; then use nitrogen as the protective gas in a pusher furnace, heat up at a heating rate of 2°C / min, and burn at a constant temperature of 720°C for 20 Hours, cooled to room temperature at a cooling rate of 4°C / min to obtain lithium iron phosphate; then pulverized by a jet mill for 10 minutes, and finally mixed for 2 hours by a double-helix conical mixer to obtain a finished product of lithium iron phosphate. The synthesized LiFeP04 / The C material and metal lithium sheet were assembled into a test battery. When discharged at a rate of 1C, the reversible specific capacity reached 153mAh / g.
Embodiment 3
[0043] Put ferrous oxalate (1750g), lithium dihydrogen phosphate (1046g), glucose (400g), niobium oxide (18g), and isopropanol (6000g) in a ball mill for 3 hours, and then grind them ultra-finely in a sand mill 3 hours, and then dried in a double-cone vacuum dryer for 4 hours to obtain the precursor. The D50 of the precursor is 0.4~0.8um; Melting at a constant temperature of 720°C for 20 hours, cooling to room temperature at a cooling rate of 4°C / min to obtain lithium iron phosphate; then pulverized by a jet mill for 10 minutes, and finally mixed for 2 hours by a double-helix conical mixer to obtain lithium iron phosphate The finished product, the synthesized LiFeP04 / C material and metal lithium sheet are assembled into a test battery, and the reversible specific capacity reaches 155mAh / g when discharged at a rate of 1C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com