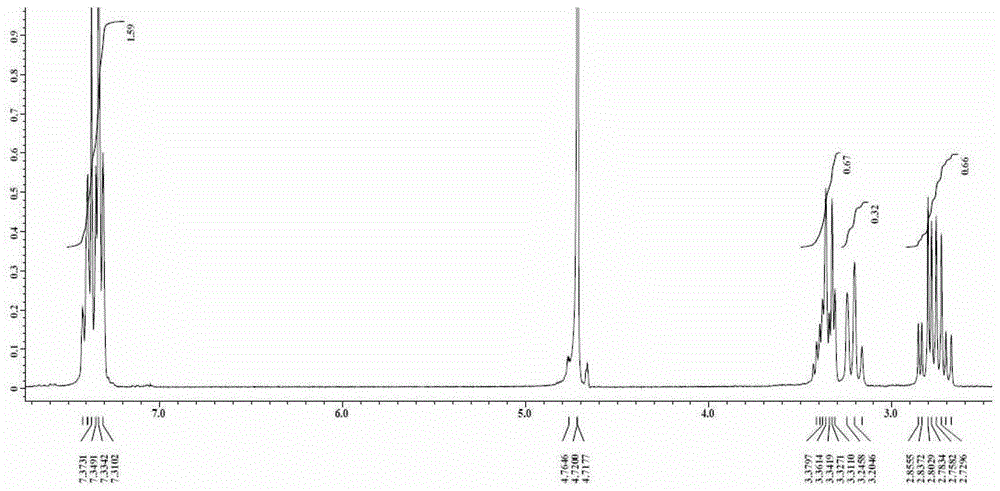
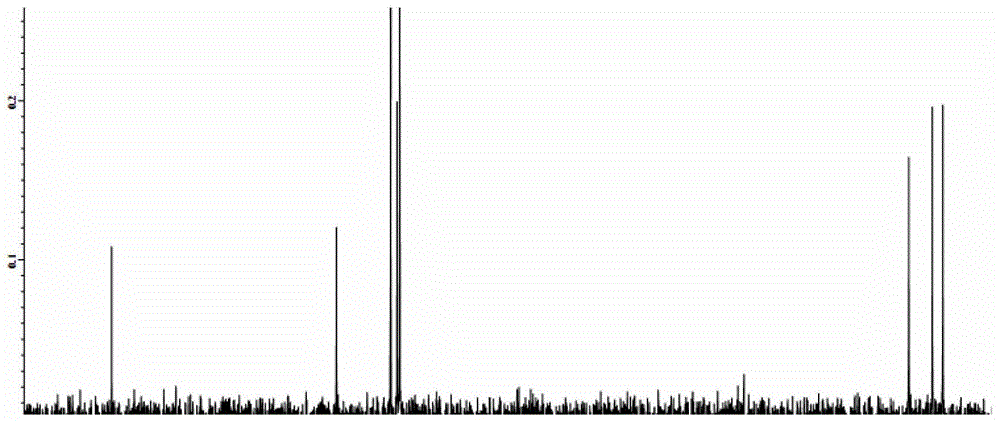
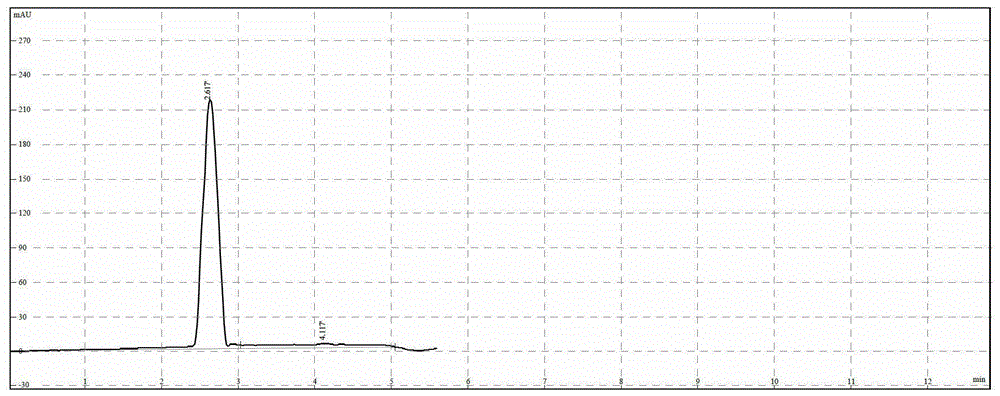
A kind of technique for preparing 3-phenyl-4-aminobutyric acid hydrochloride
A technology of aminobutyric acid hydrochloride and methyl nitrobutyrate, which is applied in the field of chemistry, can solve the problems of low overall yield of target products, many reaction steps, and difficult operation, and achieve improved hydrogenation efficiency and good reaction rate Easy and convenient effect with yield and control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Take 9.8g of methyl cinnamate and 40ml of nitromethane in a 100ml three-neck flask, stir, heat up to 50°C, then add 1.5g of triethylamine and 0.5g of anhydrous MgCl2, maintain 50°C for 10h, TLC The detection response is complete.
[0037] Distill under reduced pressure to remove excess nitromethane, then transfer the solution with a small amount of ethyl acetate, wash with 5% hydrochloric acid for 3 times, then wash with water until neutral, and then wash with anhydrous MgSO 4Dry to obtain 12.98 g of 3-phenyl-4-nitrobutyric acid methyl ester, the yield is 94.1%
[0038] Take 12.98g of methyl 3-phenyl-4-nitrobutyrate, add 200ml of ethanol, then add 0.13g of Raney nickel into the reaction system, stir, heat up to 50°C, and feed hydrogen gas (bubble), to The reaction was monitored by TLC, and the reaction time was about 8 h. After filtration, the resulting filtrate was distilled under reduced pressure to obtain 8.33 g of 3-phenylvalerolactam, with a yield of 95.7%.
[0...
Embodiment 2
[0042] Take 49g of methyl cinnamate and 200ml of nitromethane in a 500ml three-neck flask, stir, heat up to 50°C, then add 7.5g of triethylamine and 2.5g of anhydrous MgCl2, maintain 50°C for 10h, and detect by TLC The response is complete.
[0043] Distill under reduced pressure to remove excess nitromethane, then transfer the solution with a small amount of ethyl acetate, wash with 5% hydrochloric acid for 3 times, then wash with water until neutral, and then wash with anhydrous MgSO 4 Dry to obtain 64.9 g of methyl 3-phenyl-4 nitrobutyrate, the yield is 94.1%
[0044] Take 64.9g of 3-phenyl-4-nitrobutyric acid methyl ester, add 1000ml of ethanol, then add 0.65g of Raney nickel into the reaction system, stir, heat up to 50°C, and feed hydrogen gas (just bubbling), to The reaction was monitored by TLC, and the reaction time was about 12 h. After filtration, the resulting filtrate was distilled under reduced pressure to obtain 41.6 g of 3-phenylvalerolactam, with a yield of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com