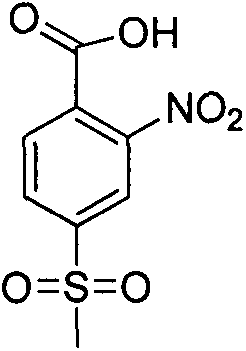
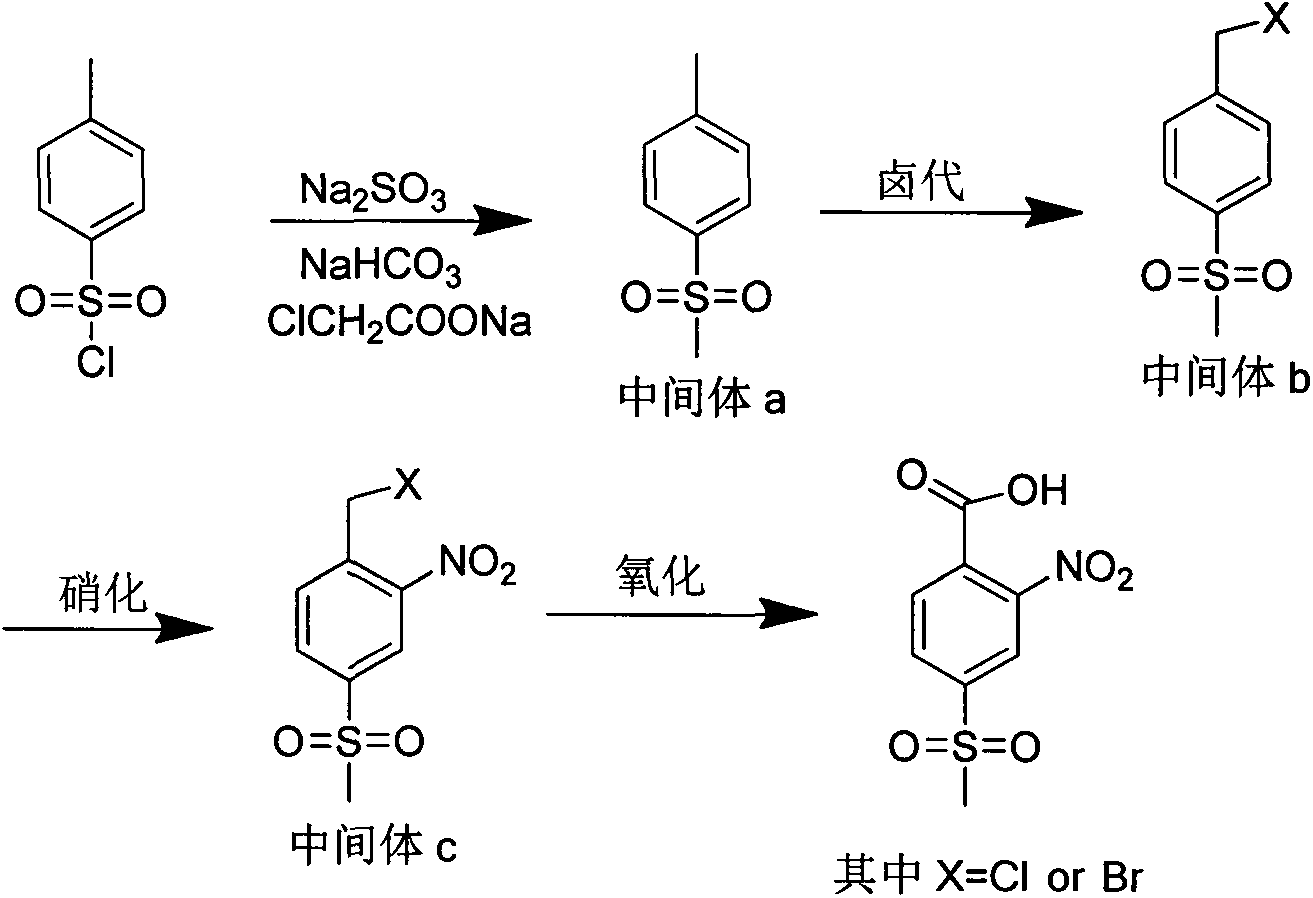
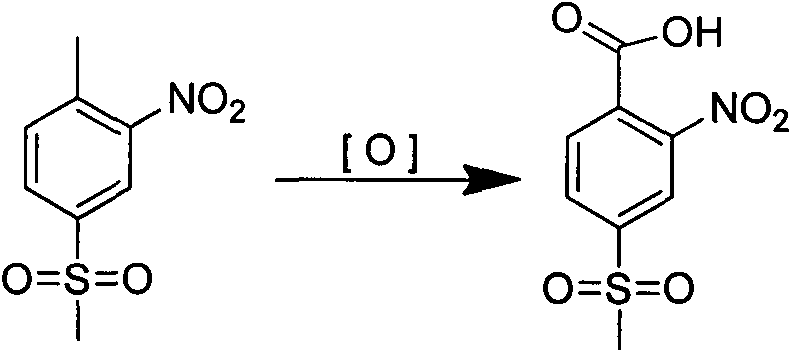Method for synthesizing 2-nitro-4-methylsulfonylbenzoic acid
A technology of methylsulfonylbenzoic acid and a synthesis method, applied in the field of organic synthesis, can solve the problems of high production cost, dangerous operation, low purity, etc., and achieve the effects of reducing the pollution of three wastes, meaning of clean production, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) Synthesis of intermediate a: 4-thiamphenicol toluene
[0021] Weigh 10.08g NaHCO 3 with 7.56g Na 2 SO 3 Put it into a 100ml four-necked bottle equipped with a thermometer, start stirring and heating, gradually raise the temperature to 75°C~80°C, add 9.53g (0.05mol) p-toluenesulfonyl chloride into the four-necked bottle in batches, keep stirring after adding 2h. Add 8.76g ClCH 2 COONa, gradually raised the temperature to about 110°C, and stirred at reflux for 18h. After the reaction is complete, cool to room temperature, adjust the pH value to neutral, and filter to obtain the crude product. After recrystallization from water / ethanol, cooling, filtration and washing with water, intermediate a7.29g (theoretical yield: 8.51g) was obtained, with a yield of 85.66%.
[0022] (2) Intermediate b: Synthesis of 4-thiamphenicol bromide
[0023] Weigh the intermediate a1.70g (0.01mol) obtained in the previous step, KBr1.55g, KBrO 3 Put 0.43g into a four-necked bottle, a...
Embodiment 2
[0029] Intermediate b: Synthesis of 4-thiamphenicol bromide
[0030] Weigh 8.51g (0.05mol) of the intermediate a4-thiamphenicol toluene, put it into a four-necked flask, add 40ml of dry carbon tetrachloride as a solvent, start stirring, heat to reflux, and slowly add 8.79g of dry liquid Br 2 , add 0.04gAlBN as a free radical reaction initiator, TLC tracking after the reaction is over, liquid separation extraction, the organic phase is saturated with NaSO 3 solution, washed with saturated brine, and rotary evaporated to obtain the crude intermediate b. Recrystallization from petroleum ether / ethanol system gave 9.95 g of intermediate b (theoretical yield: 12.46 g), with a yield of 79.86%.
Embodiment 3
[0032] Intermediate b: Synthesis of 4-thiamphenylchlorotoluene
[0033] Weigh 8.51g (0.05mol) of the intermediate a4-thiamphenicol toluene, put it into a four-necked bottle, add 40ml of dry carbon tetrachloride as a solvent, start stirring, heat to reflux state, slowly pass in dry chlorine gas, add 0.04g AlBN was used as a free radical reaction initiator, TLC followed the reaction until the raw materials disappeared, separated and extracted, and the organic phase was saturated with NaSO 3 solution, washed with saturated brine, and rotary evaporated to obtain the crude intermediate b. Recrystallization from petroleum ether / ethanol system gave 8.65 g of intermediate b (theoretical yield: 10.23 g), with a yield of 84.56%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com