Proportional near-infrared fluorescent probe as well as preparation method and application thereof
A fluorescent probe and near-infrared technology, applied in the field of probes, can solve problems such as instability, high precursor reactivity, and difficult preparation process, achieve improved selectivity, good photostability, and facilitate cell imaging analysis and measured effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
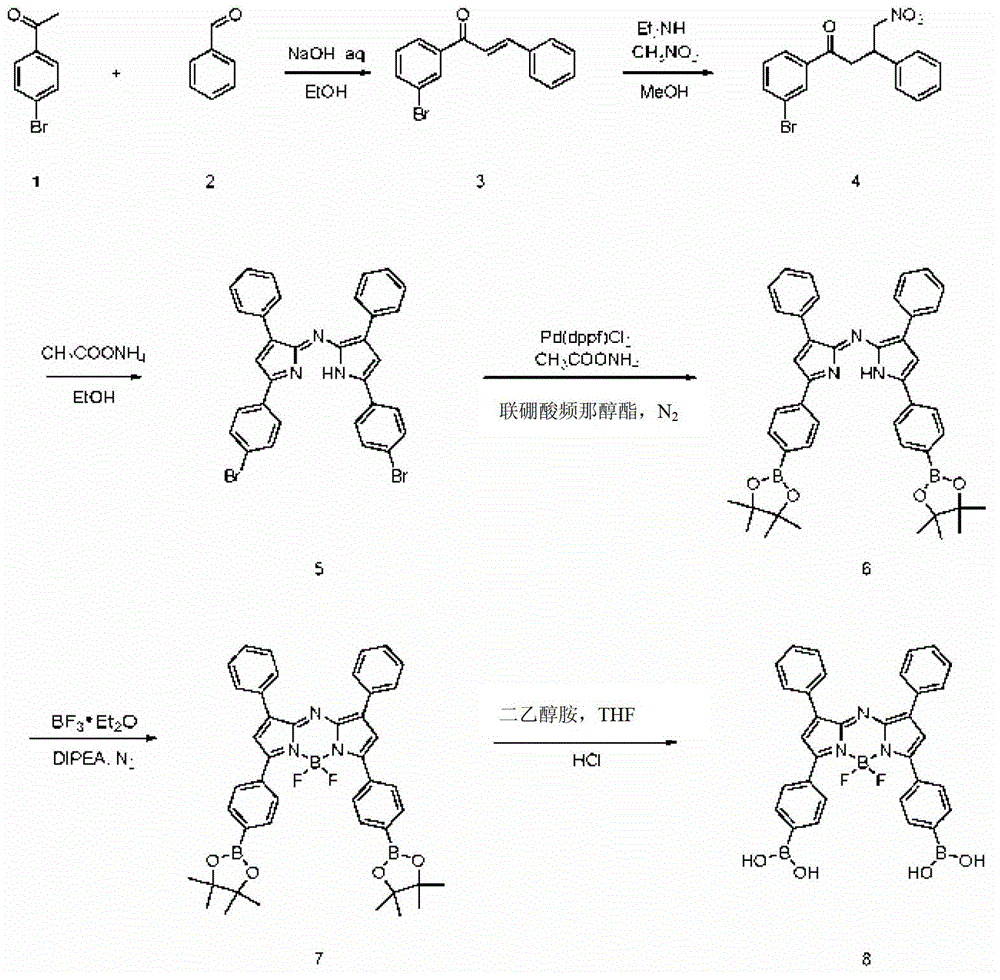
[0040] Example 1: Synthesis of Aza-bodipy-BA.
[0041] The synthetic route of Aza-bodipy-BA is as follows figure 1 shown, including the following steps:
[0042] (1) Dissolve 199.04g1mol of 4-bromoacetophenone in 2.44L of ethanol, add 2.44L of 2.5M sodium hydroxide aqueous solution, add dropwise the ethanol solution of compound 106.12g1mol of benzaldehyde at room temperature, and wait for a large amount of solid after the dropwise addition After precipitation, react at room temperature for 20 h, wash the solid with water, recrystallize with ethanol, wash the solid with ethanol and dry to obtain 229.8 g of yellow solid 1-(4-bromo)-3-propiopropenone (compound 3), yield: 80%.
[0043] Compound 3 detection: 1 H NMR (400MHz, CDCl 3 )δ (ppm): 7.92 (d, 2H, J = 10.8Hz), 7.84 (d, 1H, J = 15.68Hz), 7.67 (d, 4H, J = 14.16Hz), 7.65 (s, 1H), 7.47 (t,3H,J=5.72Hz);
[0044] (2) Compound 3 (216g, 0.75mol, 1eq), diethylamine (274.29g, 3.75mol, 5eq), nitromethane (229.5g, 3.75mol, 5eq) wer...
Embodiment 2
[0051] Example 2: H in homogeneous phase 2 o 2 detection.
[0052] All fluorescence experiments in the homogeneous detection are based on the excitation wavelength of 652nm, the excitation and emission slit widths are both 15nm, the scan rate is 100nm / min, and the attenuation is 1%.
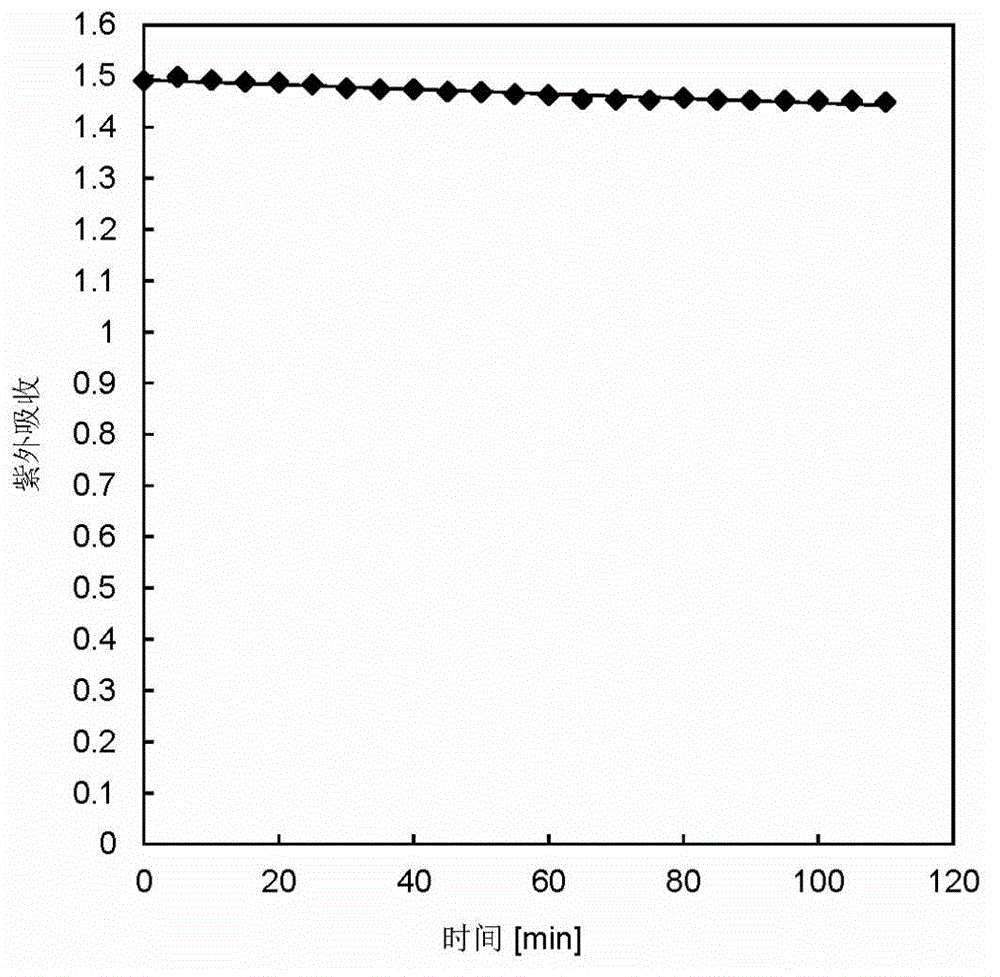
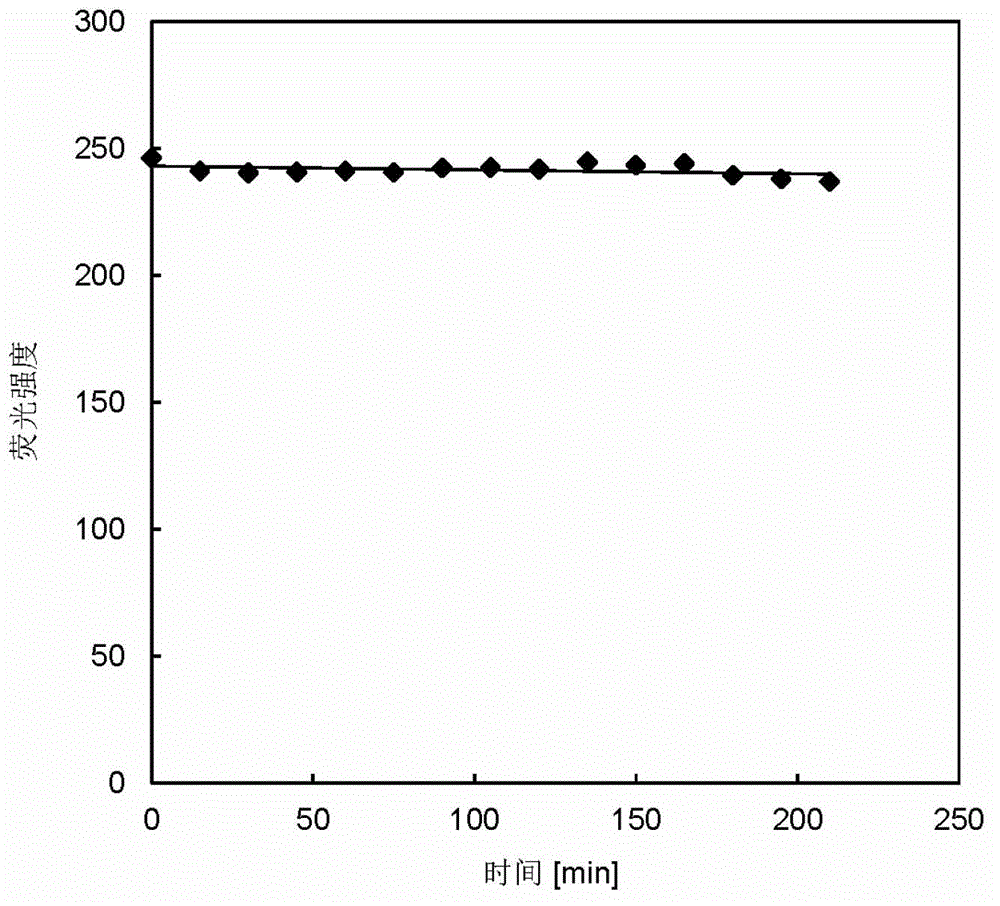
[0053] Photostability experiment: at room temperature, with an optical density of 135.9mWcm -2 , 100W mercury lamp, the wavelength range of 530 ~ 550nm green light continuous irradiation concentration of 20μM aza-bodipy-BA solution, UV absorption stability experiment. The fluorescence photostability experiment was carried out by exciting 10 μM aza-bodipy-BA every 15 minutes with an excitation wavelength of 652 nm. The results show that the quantum yield of aza-bodipy-BA in methanol is 0.60 (with Nile blue as the reference dye), and the extinction coefficient at the maximum absorption wavelength of 655nm is 74540L mol -1 cm -1 , the compound has good photostability (such as figure 2 and i...
Embodiment 3
[0057] Example 3: H in the membrane phase 2 o 2 detection.
[0058] When aza-BODIPY-BA was incorporated into polyvinyl chloride-dioctyl sebacate (PVC-DOS) films (dielectric constant ε=3 of the plasticizer DOS), its response to H 2 o 2 the response to. like Figure 8 As shown, it has an effect on H 2 o 2 The response can range from 10 -7 M to 10 -2 M, the lowest detection limit can reach 10- 8 M and the response time to fluorescence intensity ratio curve follows the first-order kinetic equation, such as Figure 9 to Figure 13 shown. In membrane phase detection, H 2 o 2 Diffusion into the membrane phase reacts with aza-bodipy-BA with a shorter response time than in the homogeneous phase. From the above results, it was suggested that the conversion of phenylboronic acid to phenol in aza-bodipy-BA was environment-related and more suitable for this reaction to occur in the organic phase, probably due to the better solubility of the probe in the organic phase. Such pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com