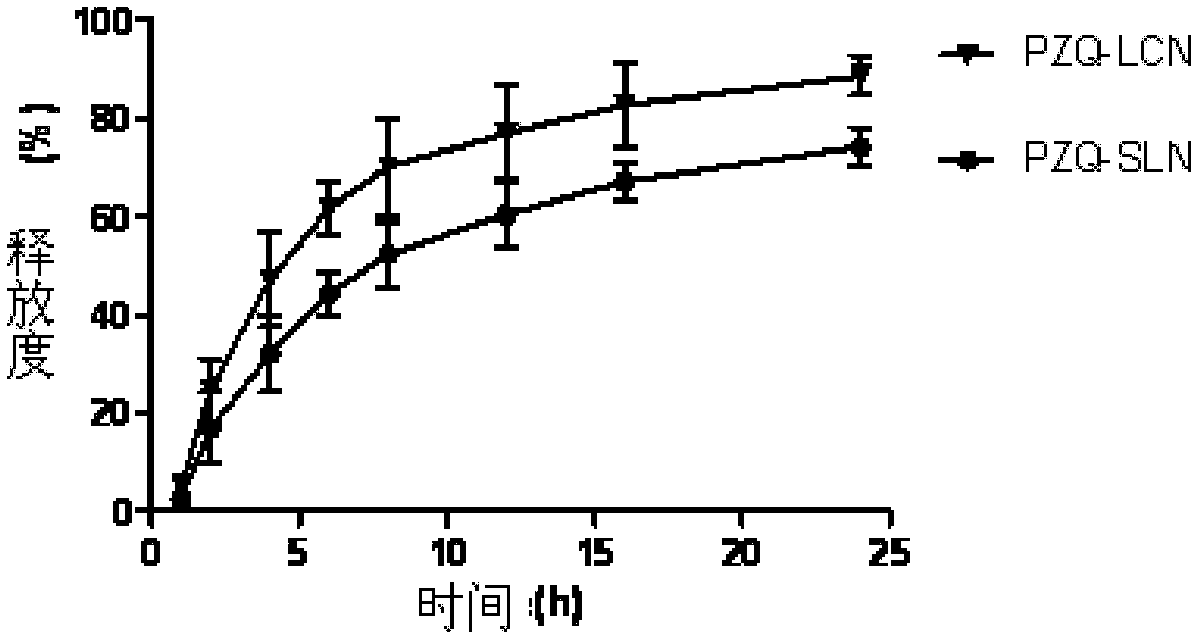
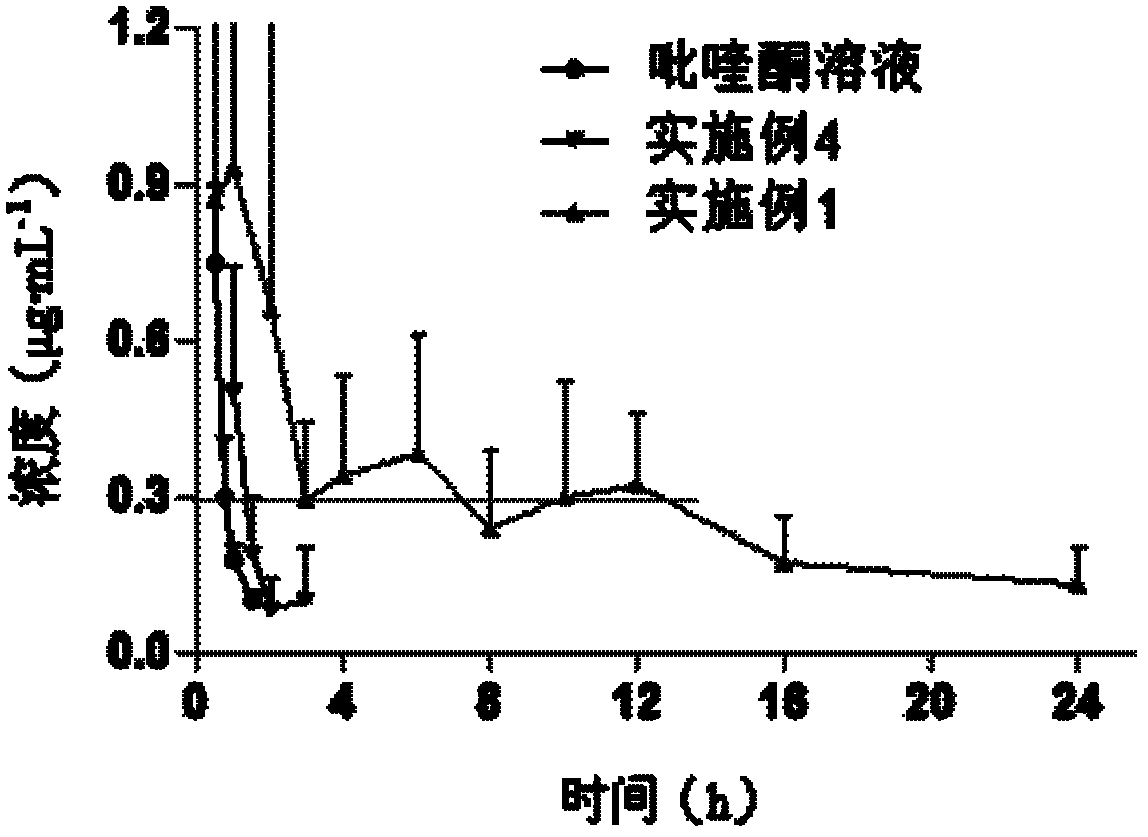
Praziquantel long-circulating nanoparticle injection and preparation method thereof
A technology for praziquantel and injection, which is applied in pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc. It can solve the problems of large particle size and strong irritation of injections, so as to achieve the effect of solving large dosage, preventing and treating schistosomiasis infection and improving curative effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
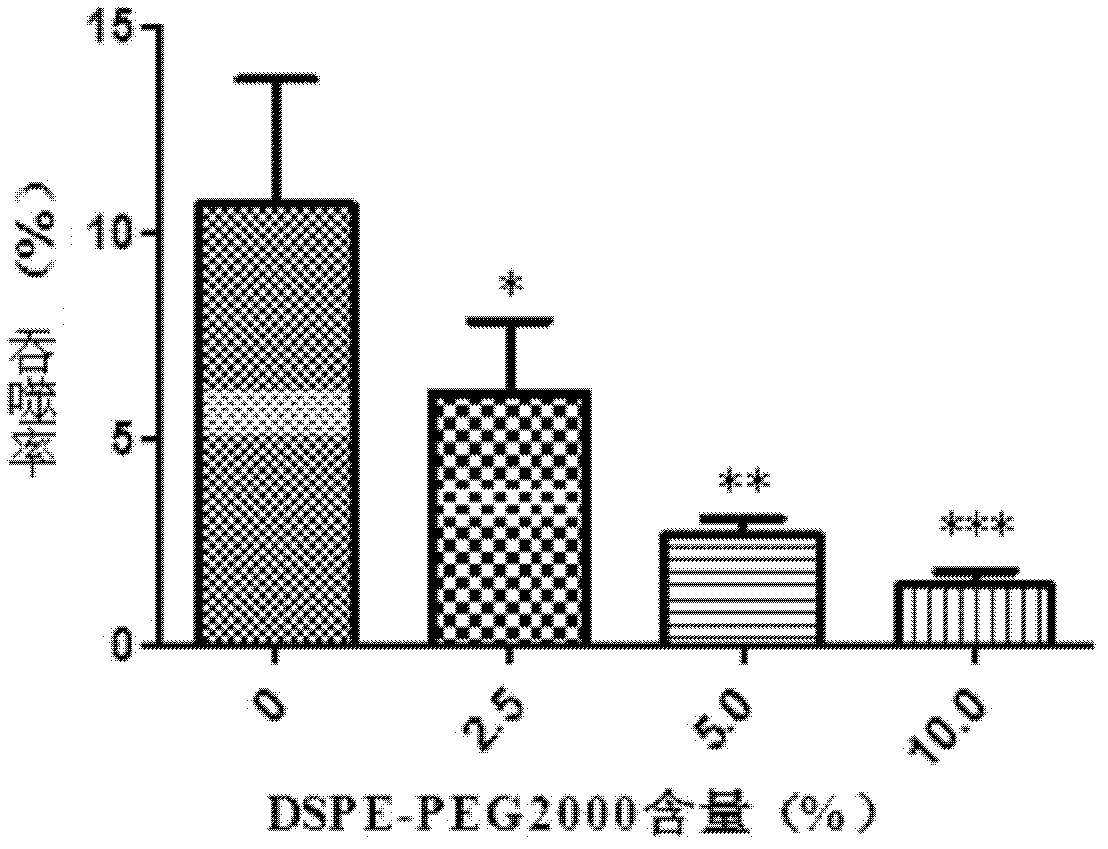
[0047] (1) Weigh 10 parts of poloxamer, 15 parts of soybean lecithin, 1 part of sodium stearate, 5 parts of DSPE-PEG2000, and 500 parts of water in a 50ml centrifuge tube. spare;
[0048] (2) Weigh 1.5 parts of each raw material praziquantel, 7.5 parts of glyceryl behenate, 7.5 parts of glyceryl monostearate, and 7.5 parts of butyl acetate in a 50ml centrifuge tube, dissolve in a 70°C water bath, and use it as the oil phase for later use;
[0049] (3) Inject the water phase into the oil phase, shear at 70°C and 8000rpm for 10 minutes, sonicate the probe for 10 minutes, and cool to room temperature with magnetic stirring to obtain praziquantel long-circulation nanoparticle injection, which contains 10% by mass Phosphatidylethanolamine-Polyethylene Glycol 2000.
Embodiment 2
[0051] (1) Weigh 10 parts of poloxamer, 15 parts of soybean lecithin, 1 part of sodium stearate, 2.5 parts of DSPE-PEG2000, and 500 parts of water in a 50ml centrifuge tube, ultrasonically dissolve the probe, and keep warm in a 70°C water bath. Standby as the aqueous phase;
[0052] (2) Weigh 1.5 parts of each raw material praziquantel, 7.5 parts of glyceryl behenate, 7.5 parts of glyceryl monostearate, and 7.5 parts of butyl acetate in a 50ml centrifuge tube, dissolve in a 70°C water bath, and use it as the oil phase for later use;
[0053] (3) Inject the water phase into the oil phase, shear at 70°C and 8000rpm for 10 minutes, sonicate the probe for 10 minutes, and cool to room temperature with magnetic stirring to obtain praziquantel long-circulation nanoparticle injection, which contains 5% by mass Phosphatidylethanolamine-Polyethylene Glycol 2000.
Embodiment 3
[0055] (1) Weigh 10 parts of poloxamer, 15 parts of soybean lecithin, 1 part of sodium stearate, 1.25 parts of DSPE-PEG2000, and 500 parts of water in a 50ml centrifuge tube, ultrasonically dissolve the probe, and keep warm in a 70°C water bath. Standby as the aqueous phase;
[0056] (2) Weigh 1.5 parts of each raw material praziquantel, 7.5 parts of glyceryl behenate, 7.5 parts of glyceryl monostearate, and 7.5 parts of butyl acetate in a 50ml centrifuge tube, dissolve in a 70°C water bath, and use it as the oil phase for later use;
[0057] (3) Inject the water phase into the oil phase, shear at 70°C and 8000rpm for 10 minutes, sonicate the probe for 10 minutes, and cool to room temperature with magnetic stirring to obtain praziquantel long-circulation nanoparticle injection, which contains 2.5% by mass Phosphatidylethanolamine-Polyethylene Glycol 2000.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com