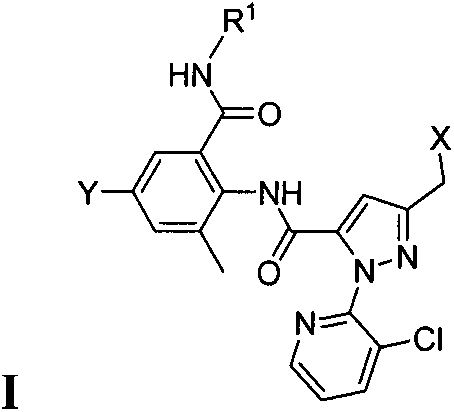
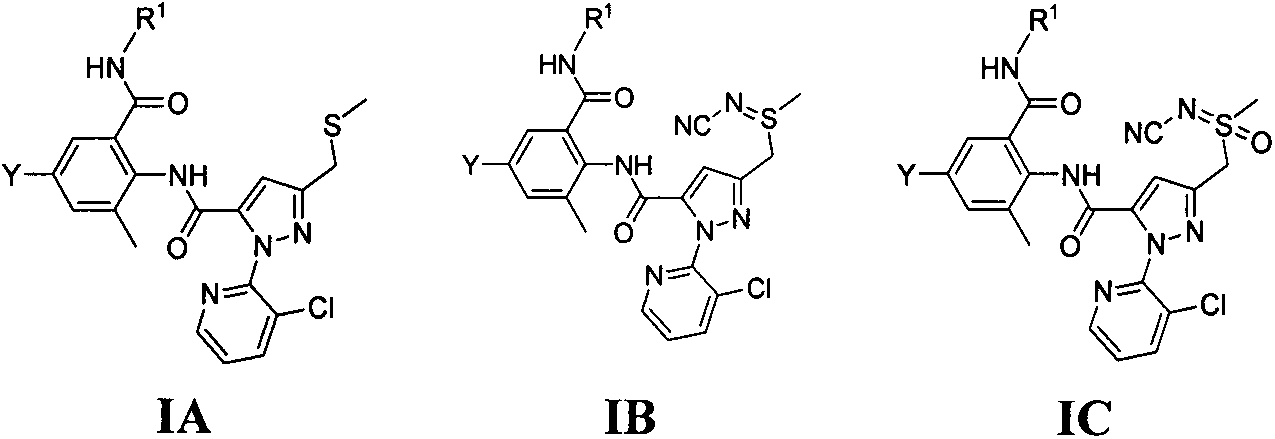
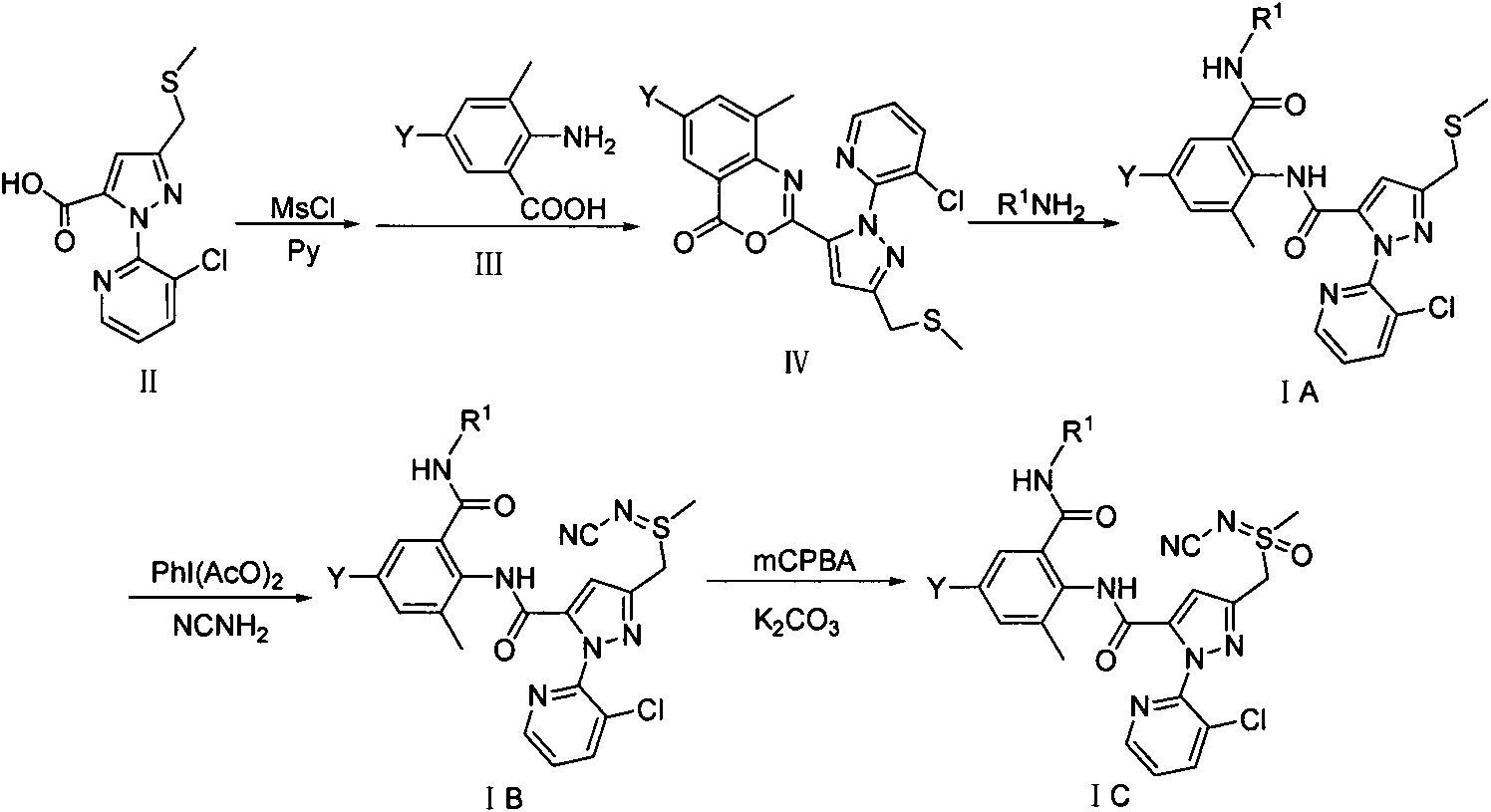
Double amide derivative containing sulfur ether and N-sulfur cyano (sulphone) imine structure and replacing pyrazolecarboxamide and preparing method and purpose thereof
A technology of cyanomethylsulfiminomethylene and pyrazole carboxamide, which is applied in the field of diamide derivatives and can solve the problems of limited number of pesticides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
[0062] The synthesis of embodiment 13-methyl-5-substituted-2-aminobenzoic acid III:
[0063] (1) Synthesis of 3-methyl-5-chloro(bromo)-2-aminobenzoic acid
[0064]
[0065] Add 20 mmoles of 3-methyl-2-aminobenzoic acid, 50 milliliters of DMF, 30 mmoles of N-chlorosuccinimide (or N-bromosuccinimide) in a 100-ml round-bottomed flask, and reflux After stirring for 3 hours, the reaction solution was poured into ice water, acidified with dilute hydrochloric acid to pH = 6, filtered, and the obtained solid was washed with a small amount of ethanol to obtain gray solid chlorinated or brominated anthranilic acid III with a yield of 83%. Y is selected from: chlorine, bromine. Among them, 3-methyl-5-chloro-2-aminobenzoic acid 1 H NMR (400MHz, DMS0-d 6 ): δ: 7.56 (d, 1H), 7.23 (d, 1H), 2.11 (s, 3H); 3-methyl-5-bromo-2-aminobenzoic acid 1 H NMR (400MHz, DMSO-d 6 ): δ: 7.88(s, 1H), 7.45(s, 1H), 2.08(s, 3H).
[0066] (2) Synthesis of 3-methyl-5-cyano-2-aminobenzoic acid
[0067] ...
Embodiment 23
[0069] The synthesis of embodiment 23-chloro-2-hydrazinopyridine
[0070]
[0071] In a 500 ml round-bottomed flask, add 50 g of 2,3-dichloropyridine, 150 ml of ethanol, and 127 g of 80% hydrazine hydrate in sequence, heat and reflux and stir for 26 hours. After TLC monitors that the reaction is complete, the reaction system is cooled to room temperature , a solid precipitated, filtered, washed the filter cake with water, and dried to obtain needle-like white crystals with a yield of 90.13%.
Embodiment 3
[0072] Example 31-(3-chloropyridin-2-yl)-3-methylthiomethylene-N-(6-methyl-2-substituted carbamoyl-4-substituted phenyl)-1H-pyrazole - Preparation of 5-formamide
[0073] (1) Synthesis of 2,2-dimethyl-4-chloromethyl-1,3-dioxolane V
[0074]
[0075] In a 1000 ml round bottom flask, add 350 ml of acetone and 2.58 g of p-toluenesulfonic acid in turn, abbreviated as p-TsO for 1 hour. After stirring and dissolving, add 37.82 ml of 1-chloroglycerin dropwise. After the dropwise addition, stir at room temperature for 12 hours. , adding triethylamine to adjust the pH to neutral, then distilling off acetone under normal pressure, and distilling under reduced pressure to collect the fraction at 46-48 degrees Celsius / 1.7kPa to obtain a colorless transparent liquid with a yield of 65.36%; 1 H NMR (400MHz, CDCl 3 ( s, 1H), 1.35 (s, 1H).
[0076] (2) Synthesis of 2,2-dimethyl-4-methylene-1,3-dioxolane VI
[0077]
[0078] In a 500 ml round bottom flask, add 22.35 g of potassium t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com