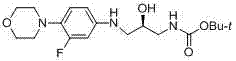
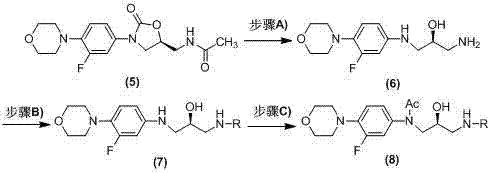
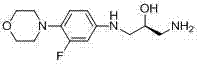
Salt of 3-amino-2-propanol acetamide compound, as well as preparation method and use thereof
A technology of propanolacetamide and compounds, which is applied in the field of medicinal chemistry, can solve problems such as difficulties, instability, and difficulty in ensuring product quality control, and achieve the effect of improving stability and inhibiting degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (2 S )-1-amino-3-[[3-fluoro-4-(4-morpholinyl)phenyl]amino]-2-propanol (6a)
[0030]
[0031] Add linezolid (0.06 mol), 150 ml of methanol, 50 ml of deionized water and lithium hydroxide (0.3 mol) into the reaction flask, heat up to reflux and stir for 8 hours, after the reaction, evaporate the solvent under reduced pressure, and the residue Dissolved with 150 ml of dichloromethane, washed with deionized water and saturated NaCl aqueous solution successively, and the organic layer was washed with anhydrous NaCl 2 SO 4 After drying, the solvent was evaporated under reduced pressure, and the residue was purified by silica gel column chromatography to obtain a white solid, m.p. 99.5-100.5°C, yield 82.6%, purity 99.8%; 1 H NMR (DMSO- d 6 , 400MHz) δ: 6.81(t, J = 9.6Hz, 1H, Ar-H), 6.41(dd, J = 2.4, 15.2Hz, 1H, Ar-H), 6.34(dd, J = 2.4, 8.8Hz, 1H, Ar-H), 5.46(t, J = 5.2Hz, 1H, Ar-NH), 4.72(brs, 1H, OH), 3.69(t, J = 4.4Hz, 4H, 2×CH 2 O), 3.49(m, 1H, CHOH), 3....
Embodiment 2
[0033] (2 R )-1-amino-3-[[3-fluoro-4-(4-morpholinyl)phenyl]amino]-2-propanol (6b)
[0034] Operation method is the same as embodiment 1 , just use linezolid with ( R )-configuration of linezolid, methyl alcohol was replaced by tetrahydrofuran, lithium hydroxide was replaced by potassium hydroxide, and the temperature was raised and refluxed for 12 hours to obtain (2 R )-1-amino-3-[[3-fluoro-4-(4-morpholinyl)phenyl]amino]-2-propanol, m.p. 99.2-100.5°C, yield 80.0%, purity 99.5%; its The structure was verified by HRMS-ESI, 1 Confirmed by H-NMR.
Embodiment 3
[0036] ( dl )-1-amino-3-[[3-fluoro-4-(4-morpholinyl)phenyl]amino]-2-propanol (6c)
[0037] Operation method is the same as embodiment 1 , just use linezolid with ( dl )-configuration of linezolid, methyl alcohol was replaced by deionized water, lithium hydroxide was replaced by sodium hydroxide, and the temperature was raised and refluxed for 10 hours to obtain ( dl )-1-amino-3-[[3-fluoro-4-(4-morpholinyl)phenyl]amino]-2-propanol, yield 88.2%, purity 99.5%; its structure was confirmed by HRMS-ESI, 1 Confirmed by H-NMR.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com