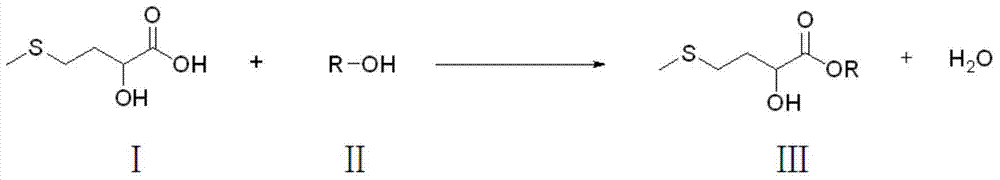
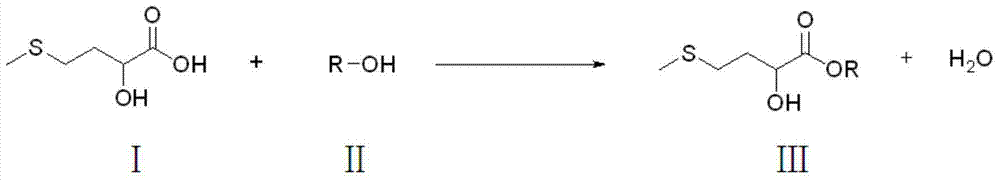
Preparation method of D,L-2-hydroxy-4-methylthio butyric ester
A technology of methylthiobutyrate and methylthiobutyric acid, which is applied in the field of preparation of D,L-2-hydroxy-4-methylthiobutyrate chemical products, can solve the problem of increasing production costs and equipment corrosion Serious, complicated process and other problems, to achieve the effects of saving production costs, reducing waste generation, and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation of embodiment one silica gel sulfonic acid
[0031] Add 1000 grams of 200-300 mesh dry silica gel into the reactor, then add 2000 grams of dichloromethane, stir at room temperature, and slowly add a certain amount of chlorosulfonic acid. After the addition was complete, stirring was continued at room temperature for 2 hours, and the hydrogen chloride gas released was absorbed with water. Stand still and remove dichloromethane to obtain silica gel sulfonic acid with a certain amount of immobilized sulfonic acid, dry and analyze the immobilized amount of sulfonic acid to obtain catalyst silica gel sulfonic acid for future use. The mass ratio of the sulfonic acid to the silica gel by weight of chlorosulfonic acid is 15-40%. The mass ratio of the chlorosulfonic acid to silica gel is 15%, 25% and 40% as shown in the table below.
[0032] catalyst
Embodiment 2
[0033] Embodiment two D, the preparation of L-2-hydroxyl-4-methylthiobutyric acid isopropyl ester
[0034] Hydroxymethionine (a mixture of 65% D,L-2-hydroxy-4-methylthiobutyric acid monomer, 20% dimer, 3% polymer and 12% water) 682 gram and 1442 grams of isopropanol were added in the three-necked flask, and the molar ratio of D, L-2-hydroxyl-4-methylthiobutyric acid and isopropanol was controlled to be 1:6, filled with nitrogen, stirred and heated, and the three-necked bottle When the temperature of the inner liquid reaches 70°C, add 60 grams of self-made silica gel sulfonic acid A catalyst, and then heat to reflux for 8 hours. After the reaction, filter out the catalyst, dry and circulate to the next esterification reaction, distill the product of the esterification reaction to remove unreacted isopropanol under normal pressure, and obtain a light yellow oily liquid after cooling. Obtain D, L-2-hydroxyl-4-methylthiobutyrate isopropyl 744.9 grams, yield is 95%, D, the purity ...
Embodiment 3
[0036] Embodiment three D, the preparation of L-2-hydroxyl-4-methylthiobutyric acid methyl ester
[0037] Hydroxymethionine (a mixture of 65% D,L-2-hydroxy-4-methylthiobutyric acid monomer, 20% dimer, 3% polymer and 12% water) 682 and 1280 grams of methanol were added to the three-necked flask, the molar ratio of D, L-2-hydroxy-4-methylthiobutyric acid to methanol was controlled to be 1:10, filled with nitrogen, stirred and heated until the temperature of the liquid in the three-necked flask reached At 60°C, 60 grams of self-made silica gel sulfonic acid B catalyst was added, and then heated to reflux for 8 hours. After the reaction, filter out the catalyst, dry and circulate to the next esterification reaction, distill the product of the esterification reaction to remove unreacted methanol under normal pressure, and obtain a light yellow oily liquid after cooling. 649.3 grams of methyl D,L-2-hydroxy-4-methylthiobutyrate were obtained, the yield was 97%, and the purity of met...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com