Method for detecting monosaccharide and preparation method for derivative reagent
A technology of derivatizing reagents and monosaccharides, which is applied in the field of medicine, can solve problems such as unsatisfactory separation results of monosaccharides, waste of biological samples, and large sample consumption, and achieve the effects of extremely small size, easy operation, and high detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Preparation of D3NMP
[0030] Weigh 5g of naphthalenehydrazine hydrochloride, put it in 60mL of water, heat it to 100°C to dissolve it completely, adjust it to 8-9 with 20% NaOH solution, obtain a solid after suction filtration, wash with water until the pH is 7, and suction filtration After vacuum drying, 3.5 g of naphthalenehydrazine was obtained.
[0031] Add 2g of naphthalenehydrazine into 20mL of absolute ethanol, stir in a water bath at 55°C until it dissolves, add 1.37g of deuterated ethyl acetoacetate dropwise, and react for 2h after the dropwise addition, then raise the temperature to 80°C and reflux for 7h, and the reaction The solution was concentrated under reduced pressure to 1 / 3 of the original volume, and recrystallized three times with 10 mL of methanol to obtain 0.9 g of D3NMP.
[0032] The calculated yield of D3NMP is 33.3%
Embodiment 2
[0033] Example 2 Liquid Phase Analysis of D3NMP Derivatives
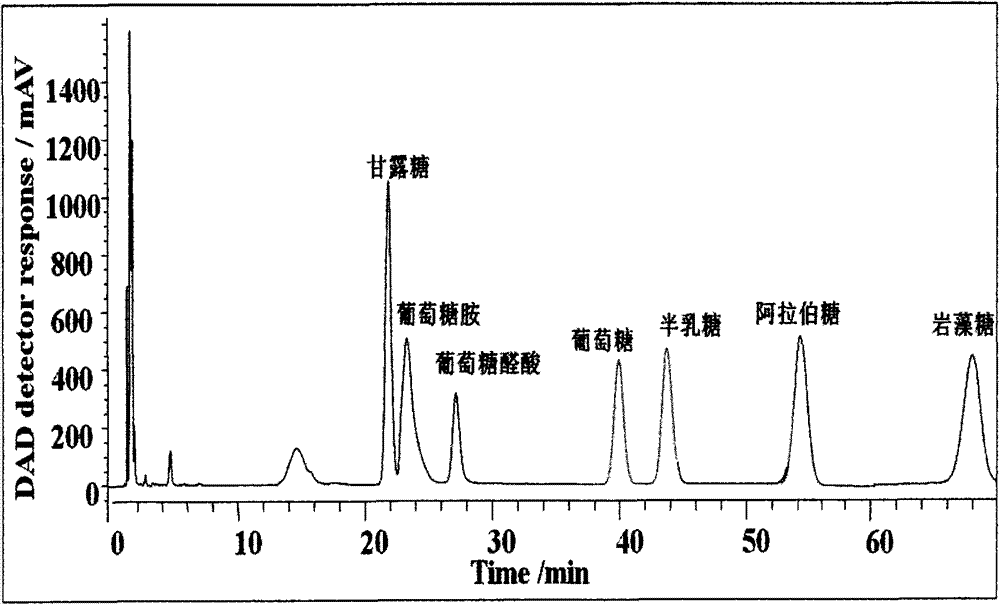
[0034] Make D3NMP into a 0.5mol / L ethanol solution, and mix glucose, glucuronic acid, glucosamine, mannose, xylose, galactose, and fucose (0.05mol / L) at 70°C in an alkaline medium of ammonia water Response 90mm. After the reaction was completed, it was extracted with chloroform three times, centrifuged, and the supernatant was taken with a final volume of 25uL for LC-MS analysis. Liquid phase conditions: liquid phase column 0.3×250mm SB-C18 column (5um, Agilent), flow rate 1mL / min, mobile phase A is acetonitrile, mobile phase B is 0.01mol / L ammonium acetate solution, and the sample volume is 2uL; Phase conditions: mobile phase 29%A15min, 29%A30min linearly increased to 33%A, 33%A15min linearly increased to 35%A, 29%A10min, 245nm detection. see results figure 1 .
[0035] The results showed that the seven monosaccharides derived from D3NMP were well separated.
Embodiment 3
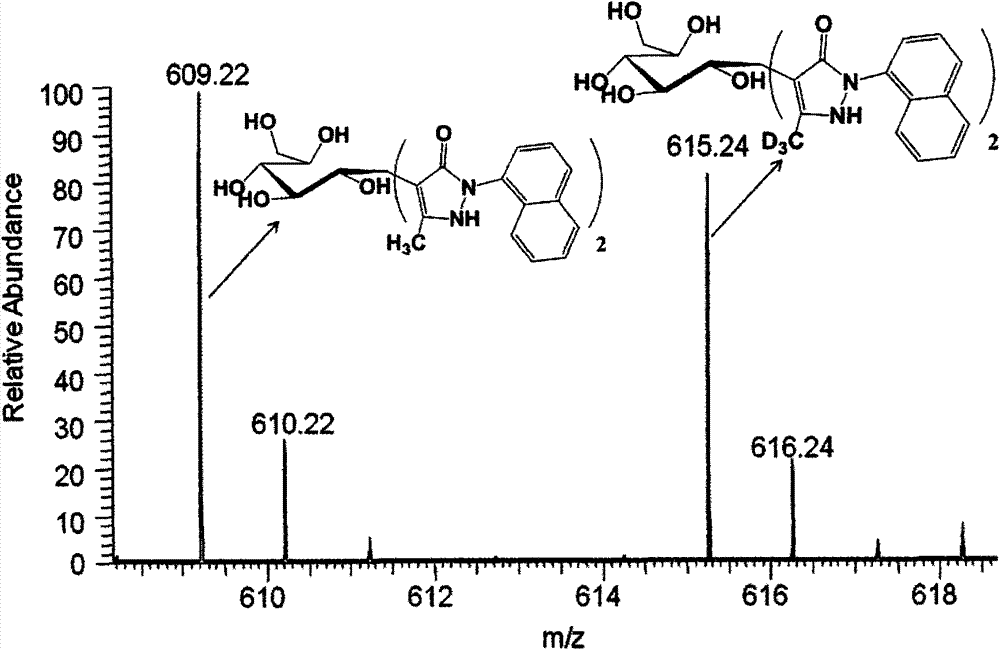
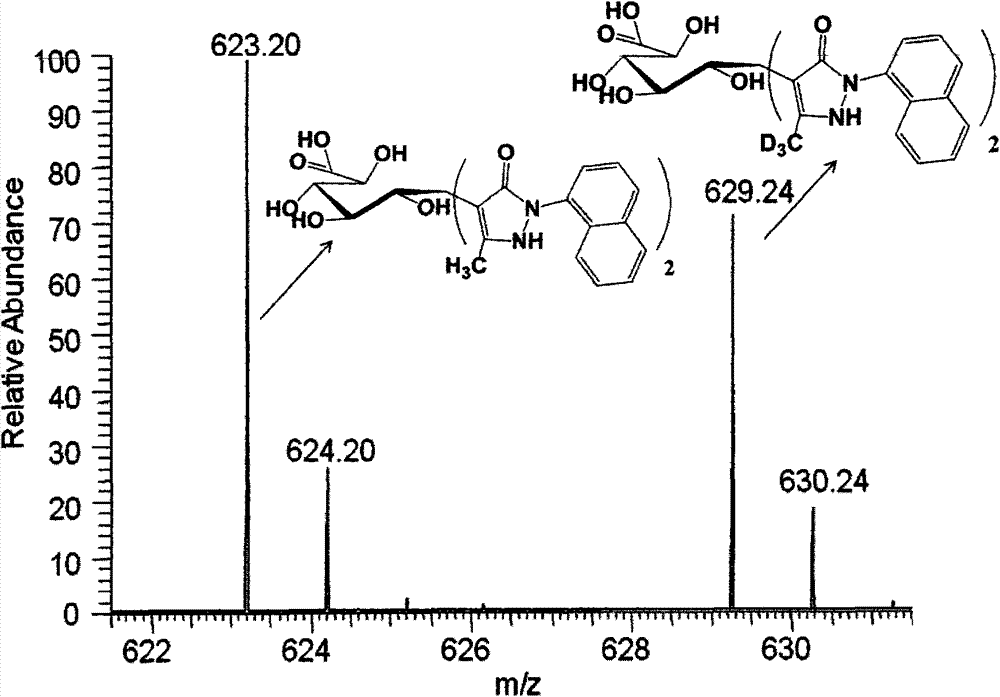
[0036] Example 3 NMP, D3NMP derived LC-MS analysis
[0037] Make NMP / D3NMP into a 0.5mol / L ethanol solution, react NMP with a known amount of glucose, glucuronic acid, and glucosamine (0.01mol / L) in a total of 10uL in an alkaline medium of ammonia water, D3NMP and the glucose to be tested, Glucuronic acid and glucosamine react at 70°C for 90 minutes. After the reaction was completed, extract with chloroform three times, centrifuge, take the supernatant, the final volume is 50uL, mix the known and unknown samples in equal volumes, and perform LC-MS analysis. Liquid phase conditions: liquid phase column 0.3×250mm SB-C18 column (5um, Agilent), flow rate 15uL / min, mobile phase A is acetonitrile, mobile phase B is 0.01mol / L ammonium acetate solution, and the sample volume is 0.02uL; Mobile phase conditions: mobile phase 27%A15min, 27%A30min linearly increased to 31%A, 31%A15min linearly increased to 34%A, 27%A10min, 245nm detection. Mass spectrometry was detected in negative ion ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com