Non-labeled ionic conjugated polyelectrolyte, synthetic method thereof and application to biological detection
A conjugated polyelectrolyte and polyelectrolyte technology, applied in chemical instruments and methods, color/spectral characteristic measurement, fluorescence/phosphorescence, etc., to achieve low cost, excellent water solubility, and good light/thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0039] In a 25 mL Schlenk tube, 9,9-bis(3,3'-dimethyl-1-propylamino)-fluorene-2,7-bisboronic acid pinacol ester (588.4 mg, 1.0 mmol), 9 ,9-bis(3,3'-dimethyl-1-propylamino)-2,7-dibromofluorene (345.8mg,0.7mmol), 4,7-dibromo-benzothiadiazole (88mg, 0.3mmol), 3.5mg tetrakis (triphenylphosphine) palladium, dissolved in 12 ml freshly distilled tetrahydrofuran and 6 ml saturated potassium carbonate mixed solution. Protected from light, heated and stirred at 85°C for 36 hours under the protection of argon. The obtained yellow polymer solution was settled in ether, the solid was collected, dissolved in ethanol, and settled again with 350 ml of ether to obtain a yellow filamentary solid, which was a neutral polymer.
[0040] Take 30 mg of neutral polymer, dissolve it in 5 ml of dimethyl sulfoxide, add 3 ml of ethyl bromide under heating and stirring at 45°C, wait for precipitation to form, add 20 ml of acetone, and add 3 ml of ethyl bromide while stirring continuously. After 40 hours...
Embodiment 1
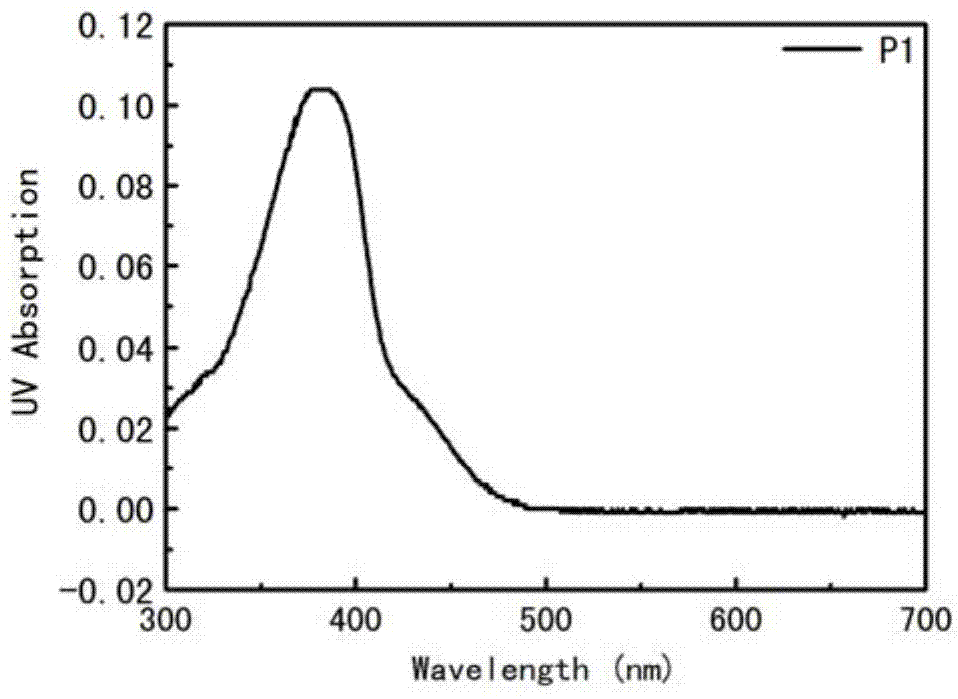
[0055] Add 4.5 μM P1 (concentration is calculated by the average number of repeating units) to the pure water system, and measure its ultraviolet absorption spectrum. which is figure 1 .
Embodiment 2
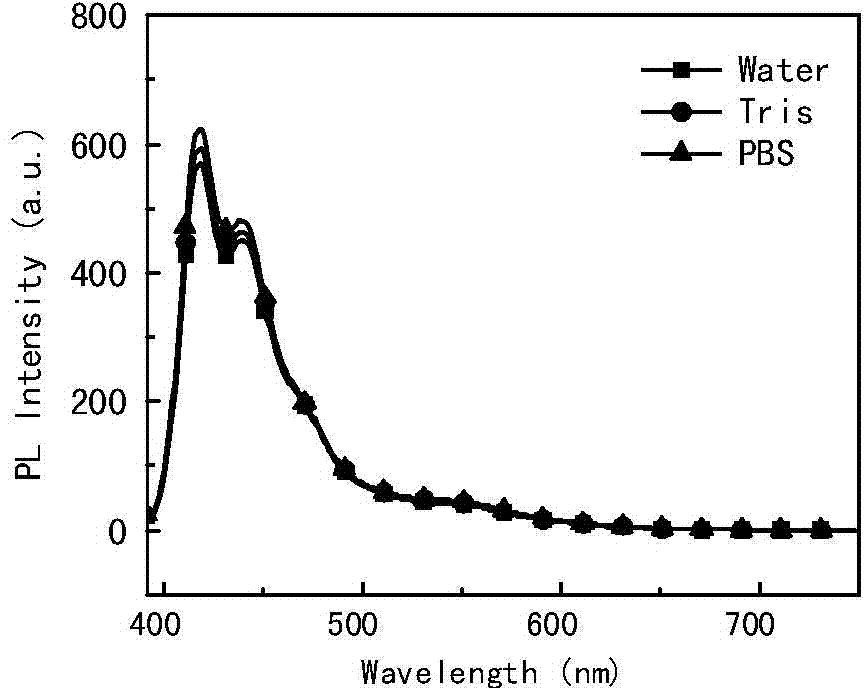
[0057] Add 4.5 μM P1 to pure water, Tris buffer solution (pH=7.4) and PBS buffer solution (pH=7.4) respectively, and measure the fluorescence emission spectrum. which is figure 2 . The excitation wavelength is 381nm, and the slit is (3;5).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com