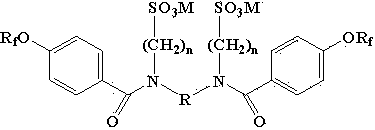
Perfluoroolefine type anionic gemini surfactant and preparation method thereof
A perfluoroalkene type, gemini surface technology, used in the preparation of sulfonates, chemical instruments and methods, organic chemistry, etc., can solve problems such as insufficient surface activity, improve decontamination and washing ability, excellent dispersibility, reduce The effect of surface tension
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
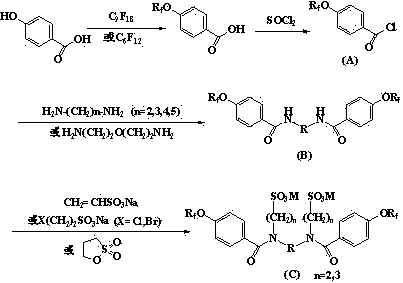
[0016] Example 1 Preparation of intermediate (B) by condensation of perfluorononenyloxybenzoyl chloride and 3-oxa-1,5-pentanediamine
[0017] 11.47g (20.2mmol) of perfluorononenyloxybenzoyl chloride obtained by chlorination of perfluorononenyloxybenzoic acid through thionyl chloride was dissolved in 30mL of acetonitrile, and 3-oxa-1,5-pentane was added dropwise A mixture of 1.04g (10.0mmol) of diamine, 2.07g (20.5mmol) of triethylamine and 30mL of acetonitrile was incubated at 50°C for 8h, the solvent was recovered under reduced pressure, stirred with water, filtered, washed with water, and dried in vacuo to obtain a light yellow solid 11.4 g, yield 94.5%.
Embodiment 2
[0018] Example 2 Preparation of fluorosurfactant by condensation of N,N'-bis(perfluorononenyloxybenzoyl)-3-oxa-1,5-pentanediamine and 1,3-propane sultone
[0019] Take 6.02g (5.0mmol) of the product obtained in Example 1, 1.34g (11.0mmol) of 1,3-propane sultone, 10mL of N,N-dimethylformamide and 20mL of water and mix, add potassium hydroxide to adjust the pH Reaction at 10,60°C for 22h. Cool, filter, wash, and dry in vacuo to obtain 7.1 g of an amber solid product with a yield of 93.4%.
[0020] Infrared spectrum characteristic peak (cm -1 ): 1643 (C=O), 1596 (benzene ring C=C), 1493 (benzene ring C=C), 1243 (S=O), 1235 (aryl-O), 1185 (C-F), 1134 (C-F ), 1129 (S=O), 1040 (S=O), there is no N-H peak around 3330. Elemental Analysis: C 42 h 28 f 34 K 2 N 2 o 11 S 2 Theoretical value C 33.07%, H 1.84%, F 42.39%; measured value C 32.90%, H 1.74%, F 42.18%. Mass spectrum: Molecular weight 1524.
Embodiment 3
[0021] Example 3 Preparation of intermediate (B) by condensation of perfluorohexenyloxybenzoyl chloride and 1,4-butanediamine
[0022] Add dropwise to 8.44g (20.2mmol) of perfluorohexenyloxybenzoyl chloride obtained by chlorination of perfluorohexenyloxybenzoic acid through thionyl chloride, 2.79g (20.2mmol) of anhydrous potassium carbonate and 40mL of tetrahydrofuran The mixed solution of 0.88g (10.0mmol) of 1,4-butanediamine and 30mL of tetrahydrofuran was refluxed for 8h, the solvent was recovered under reduced pressure, stirred with water, filtered, washed with water, and dried in vacuo to obtain 7.9g of light yellow solid with a yield of 88.9%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
| surface tension | aaaaa | aaaaa |
| surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com