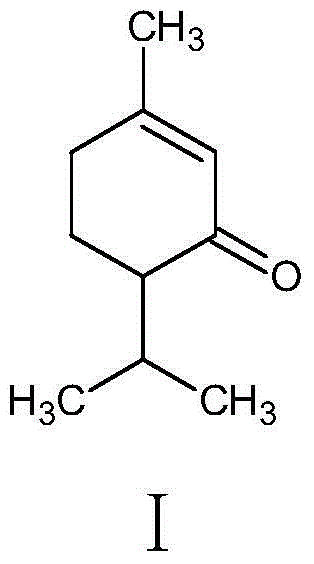
A kind of synthetic method of piperonone
A synthetic method and technology of piperonone, applied in the field of synthesis of piperonone, can solve the problems of difficult starting materials, toxicity, high cost, etc., and achieve the effects of improving the three wastes, improving operating conditions, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
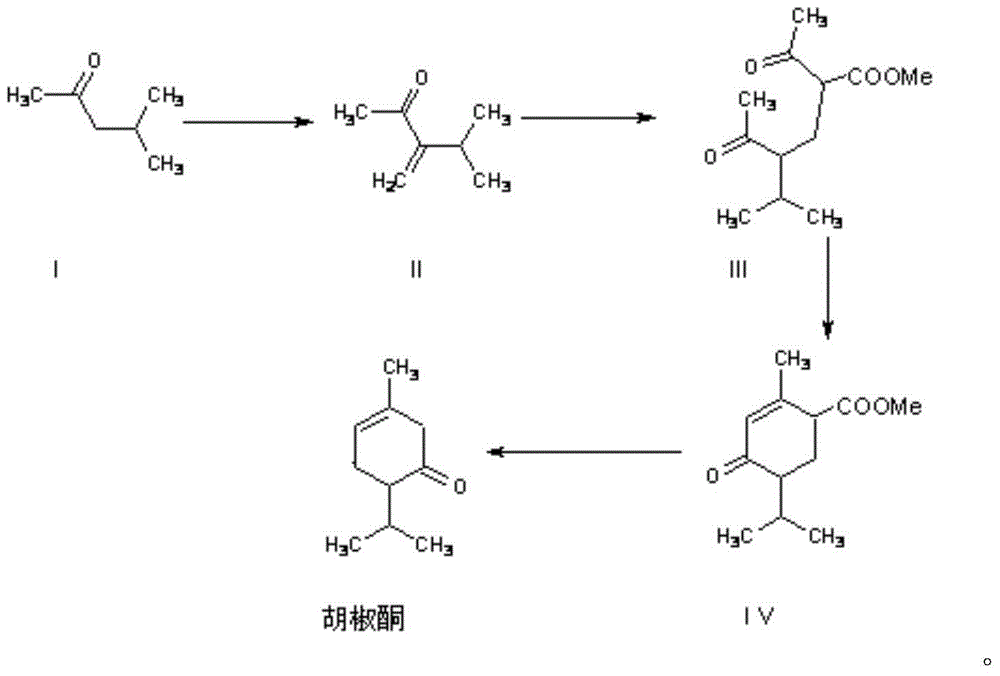
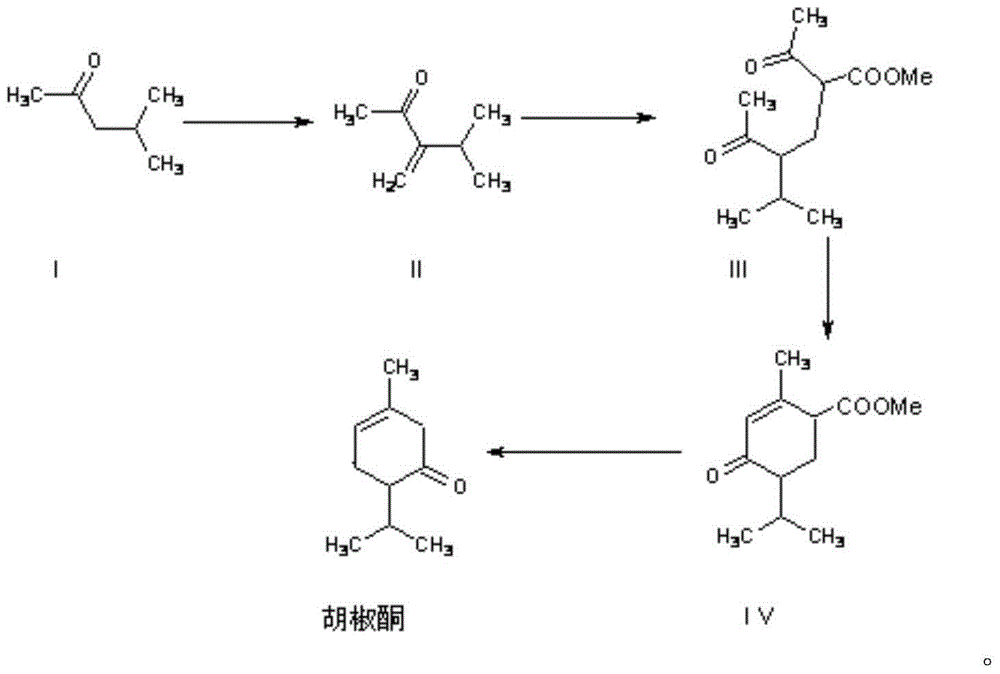
[0030] Synthesis of 3-isopropyl-3-buten-2-one (II)
[0031] Install an electric stirring device on a 2000ml four-necked bottle and place it in a water bath, and install a thermometer and a gas absorption device.
[0032] Add 1000g of methyl isobutyl ketone to a 2000ml three-necked flask under stirring, cool in a cold bath to -5°C, and pass in dry hydrogen chloride for 20 minutes while stirring; then pass in formaldehyde produced by the decomposition of 68g of paraformaldehyde for about 3 hours finished. Keep hydrogen chloride flowing slowly and steadily. Stir for 16 hours. The product was washed with 10% sodium hydroxide solution, 200mlx3; washed with 8% sodium sulfate aqueous solution until neutral; dried over anhydrous sodium sulfate.
[0033] 30g of white oil and 30g of quinoline were added to the material; excess methyl isobutyl ketone was distilled off by water ring pump vacuum distillation. Collect 65-68°C / 2000Pa. Yield 47%.
Embodiment 2
[0035] Synthesis of 2-acetyl-4-isopropyl-5-oxo-hexanoic acid methyl ester (III)
[0036] Install an electric stirring device on a 1000ml three-necked flask and place it in a water bath, add 126g of methyl acetoacetate, 200g of methanol, and 6g of 30% methanol solution of sodium methoxide; keep the reaction temperature below 25°C in the water bath; add 112g of 3-iso Propyl-3-buten-2-one was added dropwise in about 2 hours. The reaction was continued for 18 hours while maintaining the reaction temperature below 30°C. Gas chromatography detected that the reaction was complete. Add 2.4 g of glacial acetic acid. Methanol was recovered at normal pressure; 85-90°C / 1mm was collected by vacuum distillation to obtain 2-acetyl-4-isopropyl-5-oxo-hexanoic acid methyl ester, 178.5g (75%).
Embodiment 3
[0038] Synthesis of Methyl Piperonone Carboxylate (IV)
[0039] Install an electric stirring device on a 2000ml three-necked flask and place it in a water bath, add 10g of 30% sodium methoxide methanol solution, 600g of toluene, and distill out the methanol; cool to 50°C, add dropwise 45g of 2-acetyl-4-isopropyl - Methyl 5-oxo-hexanoate, the dropwise addition was completed in about 3 hours; continue to stir at 50-55°C for 12 hours, and the reaction was detected by gas chromatography until the reaction was complete. Cool, add 3.4g of glacial acetic acid to neutralize, and wash with 100ml of saturated brine. The upper oily phase was separated. The oil phase was recovered toluene under reduced pressure with a water ring pump; the crude product was 42g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com