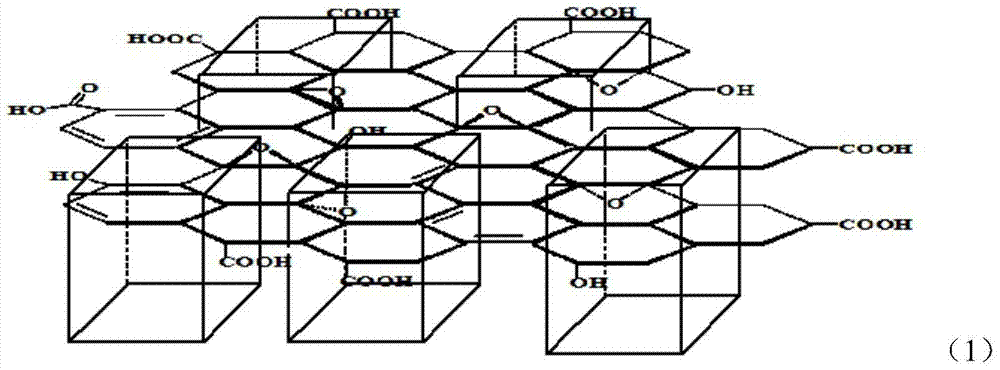
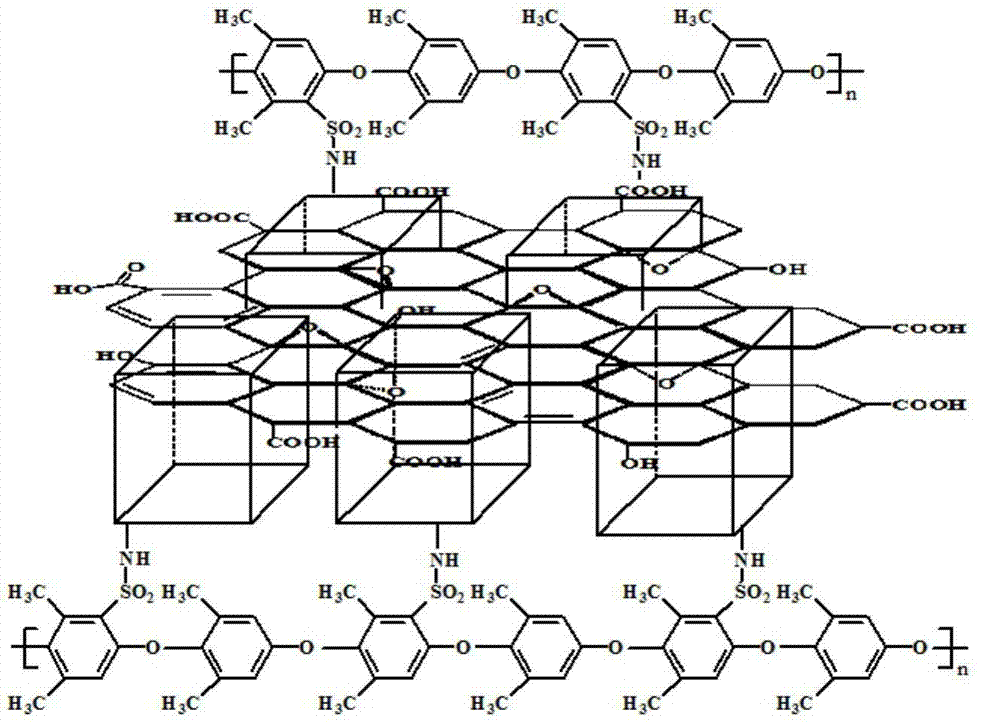
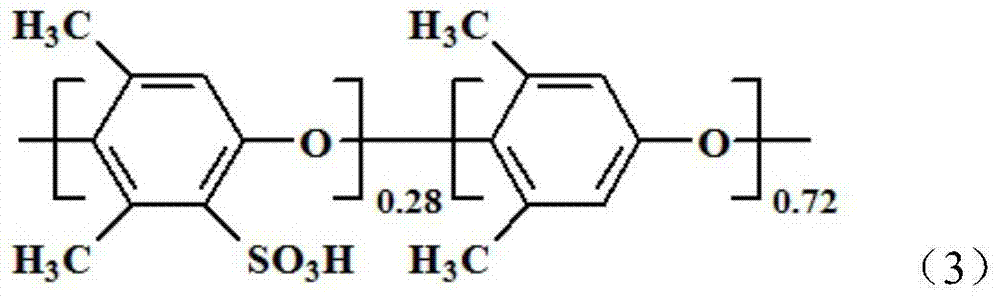
Method for preparing high-temperature proton exchange membrane
A technology of proton exchange membrane and high temperature, which is applied in the field of preparation of high temperature proton exchange membrane, can solve the problems of affecting proton conductivity, affecting proton conductivity, proton conductivity reduction, etc., and achieves excellent high temperature proton conductivity and oxidation resistance, Continuous and long-distance proton conduction, the effect of enhancing the ability of proton conduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] This embodiment prepares the high temperature proton exchange membrane according to the following steps:
[0035] (1) Preparation of graphene oxide-metal organic framework: Ultrasonic dispersion of 0.03 g graphene oxide in 10 mL of water-ethanol mixture (volume ratio of water and ethanol is 1:1) to obtain mixture A, and then metal organic framework The precursor synthesis of the framework (0.1g basic zinc carbonate, 0.1g oxalic acid and 0.4g 3-amino-1,2,4-triazole) was ultrasonically dispersed in the obtained mixture A, and reacted for 72 hours at 180°C to obtain the product Wash with water and ethanol three times respectively, and then dry at 60° C. (dry for 4 hours) to obtain a brown-black graphene oxide-metal-organic framework coexistence body.
[0036] (2) Preparation of N-methylpyrrolidone solution of sulfonylchlorinated polyphenylene ether: Dissolve 0.2g of sulfonated polyphenylene ether in 1mL (0.9445g) of N,N-dimethylformamide, and slowly Add 0.5mL (0.838g) of ...
Embodiment 2
[0043] This embodiment prepares the high temperature proton exchange membrane according to the following steps:
[0044] (1) Preparation of graphene oxide-metal organic framework: Ultrasonic dispersion of 0.06 g graphene oxide in 10 mL of water-ethanol mixture (volume ratio of water and ethanol is 1:1) to obtain mixture A, and then metal organic framework The precursor synthesis of the framework (0.1g basic zinc carbonate, 0.1g oxalic acid and 0.4g 3-amino-1,2,4-triazole) was ultrasonically dispersed in the obtained mixture A, and reacted for 72 hours at 180°C to obtain the product Wash with water and ethanol three times respectively, and dry at 60°C (dry for 4 hours) to obtain a brown-black graphene oxide-metal organic framework coexistence body.
[0045] (2) Preparation of N-methylpyrrolidone solution of sulfonylchlorinated polyphenylene ether: Dissolve 0.2g of sulfonated polyphenylene ether in 1mL (0.9445g) of N,N-dimethylformamide, and slowly Add 0.5mL (0.838g) of thionyl...
Embodiment 3
[0052] (1) Preparation of graphene oxide-metal organic framework: Ultrasonic dispersion of 0.09 g graphene oxide in 10 mL of water-ethanol mixture (volume ratio of water and ethanol is 1:1) to obtain mixture A, and then metal organic framework The precursor synthesis of the framework (0.1g basic zinc carbonate, 0.1g oxalic acid and 0.4g 3-amino-1,2,4-triazole) was ultrasonically dispersed in the obtained mixture A, and reacted for 72 hours at 180°C to obtain the product Wash with water and ethanol three times respectively, and then dry at 60° C. (dry for 4 hours) to obtain a brown-black graphene oxide-metal-organic framework coexistence body.
[0053] (2) Preparation of N-methylpyrrolidone solution of sulfonylchlorinated polyphenylene ether: Dissolve 0.2g of sulfonated polyphenylene ether in 1mL (0.9445g) of N,N-dimethylformamide, and slowly Add 0.5mL (0.838g) of thionyl chloride dropwise, a white precipitate is formed, wash and filter with water, and dry at 60°C (dry for 2 ho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com