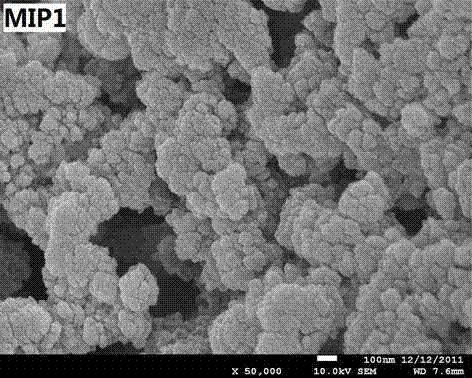
Preparation method for enrofloxacin molecular imprinting monolithic column
A molecular imprinting and enrofloxacin technology, which is applied in material separation, analysis materials, measuring devices, etc., can solve the problems of unstable polymer backbone, non-recyclable, low separation efficiency, etc., and achieve stable and rapid mass transfer capability, Strong affinity and high separation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] In a Erlenmeyer flask, weigh 0.5 mmol of enrofloxacin, add 0.4 mL of toluene, 1.6 mL of dodecanol and 0.4 mL of polyethylene glycol-200 (PEG-200), after ultrasonic mixing, add the functional monomer methyl Acrylic acid (MAA) 1.0 mmol and hydroxyethyl methacrylate (HEMA) 1.0 mmol were allowed to interact for 2 h to form a complex. Then add 5.0 mmol of ethylene glycol dimethacrylate (EDMA) and 2.5 mmol of trimethylolpropane trimethacrylate (TMPTA) as crosslinking agents, and 30 mg of initiator azobisisobutyronitrile (AIBN), The solution was ultrasonically degassed for 10 min, deoxygenated by nitrogen for 20 min, and then the mixture was injected into a stainless steel chromatographic column (50 mm × 4.6 mm) and sealed. Stand still vertically in a 55°C water bath for 24 h. After the reaction is finished, cool to room temperature, take out the chromatographic column synthesized, remove the sealing head, connect it to the liquid chromatography pump, and remove the porogen a...
Embodiment 2
[0044] Example 2 Performance testing of molecularly imprinted monolithic columns
[0045] (1) Detection method of dynamic adsorption capacity: connect the molecularly imprinted monolithic columns prepared in Examples and Comparative Examples to a chromatograph, use acetonitrile / water (25 / 75, v / v) as mobile phase at 0.8 mL / min Rinse the column at flow rate and monitor at 280 nm until the baseline is stable; then withdraw the monolithic column from the HPLC system and replace with acetonitrile / water (25 / 75, v / v) containing enrofloxacin 1.0 mg / mL The solution is the mobile phase, fully flush the pipeline from the mobile phase inlet to the chromatographic column; then connect the monolithic column back to the chromatographic system, and continue to use the acetonitrile / water (25 / 75) solution of enrofloxacin as the mobile phase, still Perform chromatographic experiments at a flow rate of 0.8 mL / min, and record the detection signal to obtain dynamic breakthrough. The dynamic adsorp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com