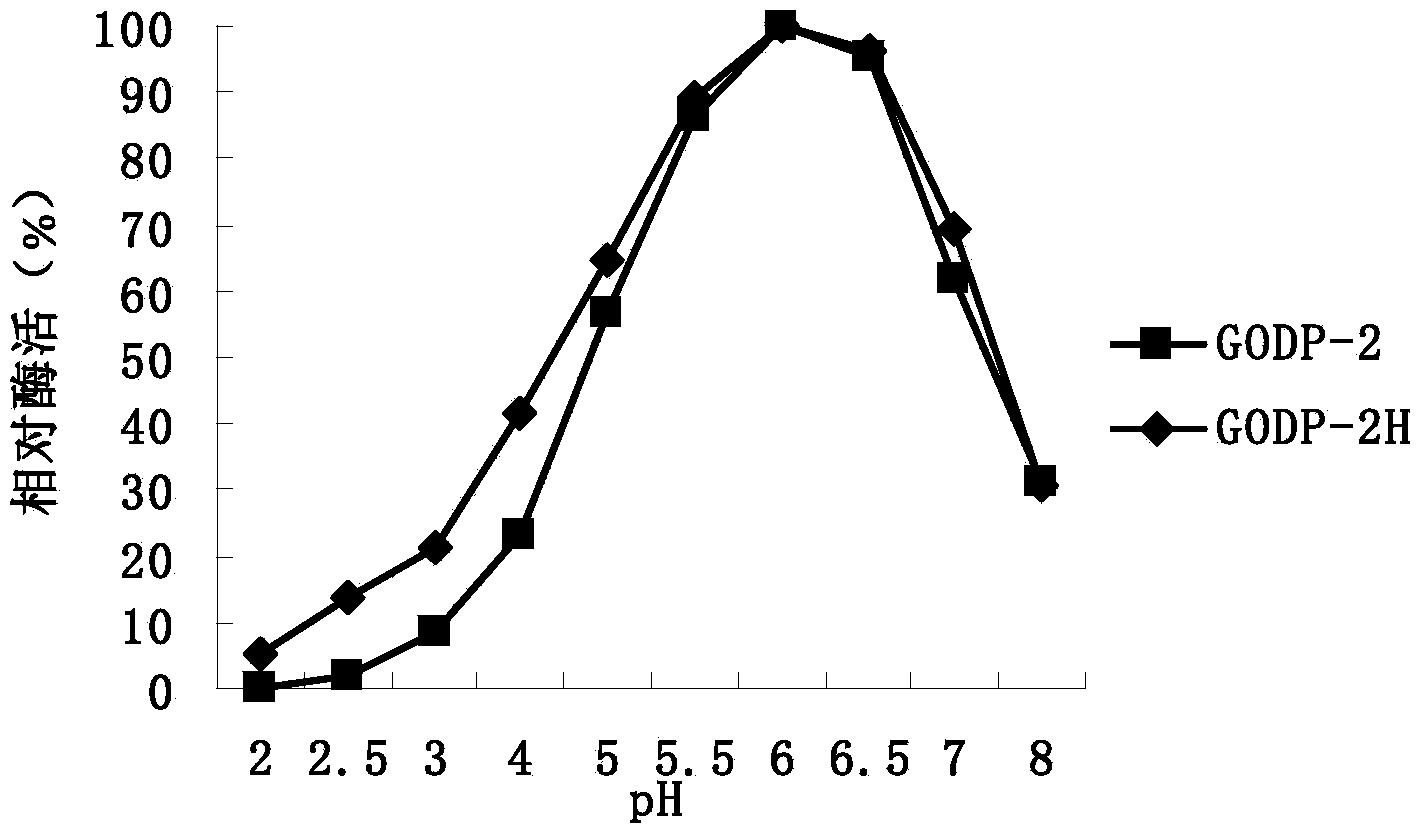Glucose oxidase mutant and application thereof
A technology of glucose oxidase and mutants, which is applied in the field of glucose oxidase mutants, can solve the problems of restricting wide application, high production costs, and the ability of glucose oxidase expression to meet industrial production, so as to improve operational performance, internal Tissue texture structure is good, the effect of strengthening muscle strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1 Synthesis and amplification of glucose oxidase mutant gene
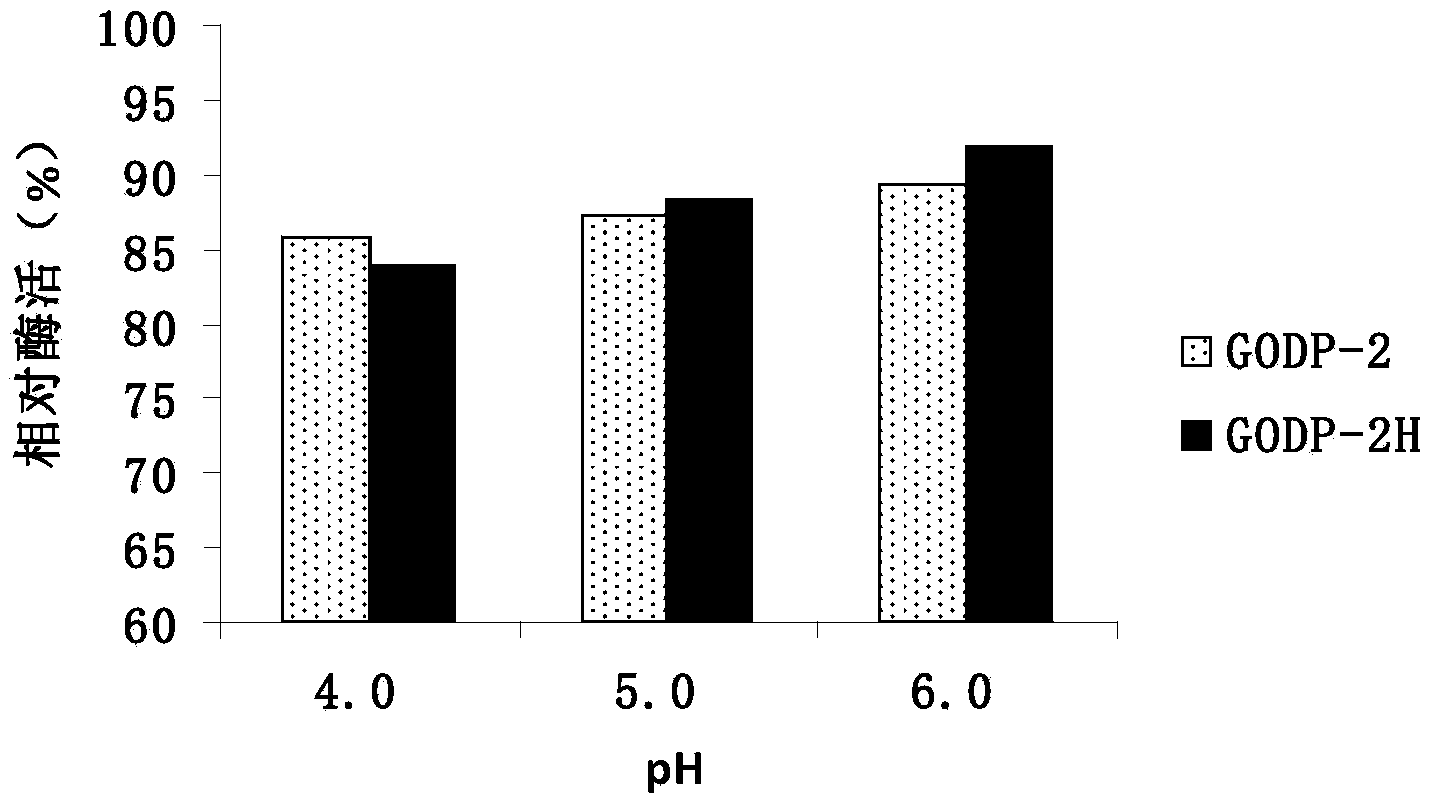
[0021] In order to improve the heat resistance of glucose oxidase GODP-2 (amino acid sequence: SEQ ID NO: 1) derived from Penicillium decumbens, a large number of mutations of the enzyme were screened by directed evolution technology, and the PCR primer GODP-2 was designed -F, GODP-2-R are as follows:
[0022] GODP-2-F: GGC GAAT TCTACTTGCCAGCACAGCAAATTGATGT (the underline is the restriction endonuclease EcoR I recognition site)
[0023] GODP-2-R: ATA GCGGCCGC TTAAGCAGACTTGGCGTAGTCATCC (the underline is the restriction endonuclease Not I recognition site)
[0024] Using the GODP-2 wild-type gene as a template, the GeneMorph II random mutation PCR kit (Stratagene) was used for PCR amplification with the above primers, and the pET- 21a vector was connected, transformed into E. coli BL21(DE3), spread on LB+Amp plate, and cultured upside down at 37°C. After the transformants appeared, pick them o...
Embodiment 2
[0029] The construction of embodiment 2 Pichia pastoris engineering strain
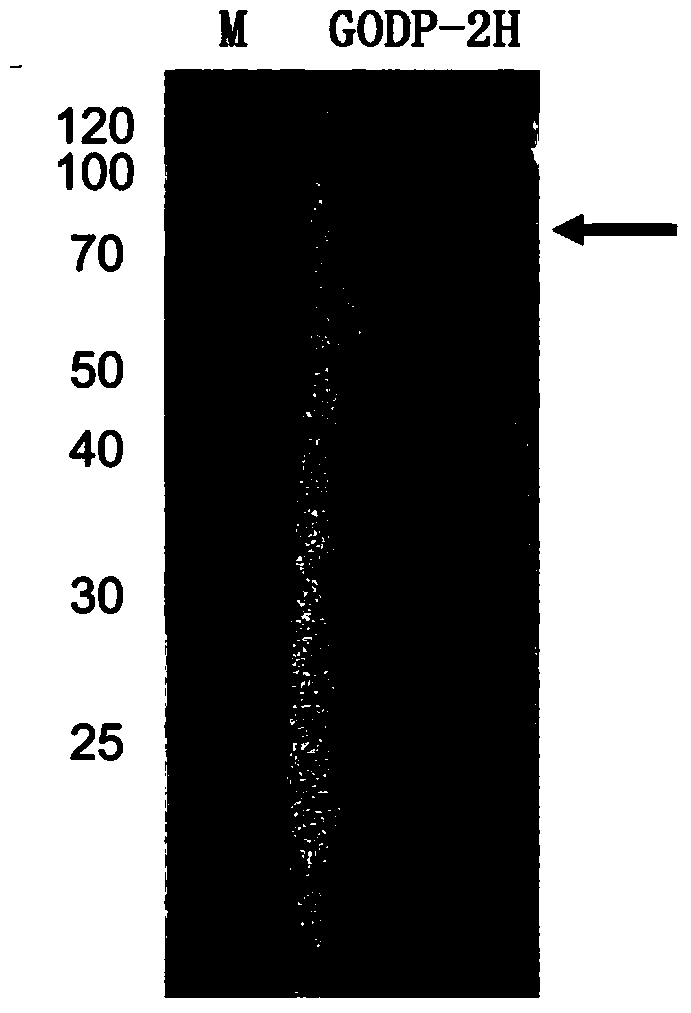
[0030] The fragment of glucose oxidase GODP-2H cloned above was connected to the expression vector pPIC9K through the EcoRI and NotI sites to construct the expression vector pPIC9K-P2H.
[0031] The expression plasmid pPIC9K-P2H was linearized with SalI, and the linearized fragment was transformed into Pichia pastoris GS115 by electroporation, and the recombinant strain of Pichia pastoris GS115 / pPIC9K-P2H was obtained by screening on the MD plate, and YPD with different concentrations of geneticin Plate screening for high copy transformants.
[0032] One of the transformants was named Pichia pastoris GODP-2H (Pichia pastoris GODP-2H), transferred to BMGY medium, 30°C, 250rpm shaking culture for 1d; then transferred to BMMY medium, 30°C, 250rpm shaking Cultivate; add 0.5% methanol every day to induce expression for 4 days; centrifuge to remove the bacteria to obtain the fermentation supernatant contai...
Embodiment 3
[0034] Embodiment 3 fermentation verification
[0035] Fermentation of Pichia pastoris GODP-2 and Pichia pastoris GODP-2H were carried out in 10L fermenter respectively. The medium formula used for fermentation was: calcium sulfate 1.1g / L, potassium dihydrogen phosphate 5.5g / L, dihydrogen phosphate Ammonium 55g / L, potassium sulfate 20.3g / L, magnesium sulfate 16.4g / L, potassium hydroxide 1.65g / L, defoamer 0.05%.
[0036] Fermentation process: pH value 5.0, temperature 30°C, stirring rate 300rpm, ventilation rate 1.0-1.5 (v / v), dissolved oxygen controlled above 20%.
[0037] The entire fermentation process is divided into three stages: the first stage is the bacterial cell culture stage, the seeds are inserted at a ratio of 7%, and cultured at 30°C for 24-26 hours, marked by the completion of glucose; the second stage is the starvation stage, when the glucose is replenished After that, do not feed any carbon source. When the dissolved oxygen rises above 80%, it means the end of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com