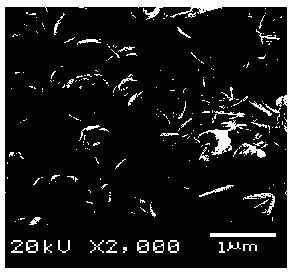
Lithium iron phosphate with controllable morphology and preparation method of lithium iron phosphate
A technology of lithium iron phosphate and morphology, which is applied in the field of energy storage secondary lithium battery materials, can solve the problems of inconspicuous morphology, unstable voltage, and low uniformity, and achieve obvious morphology, high production efficiency, The effect of low equipment investment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1) Mix the lithium source, iron source, and phosphorus source at a molar ratio Li:Fe:P=1:1:1, add an appropriate amount of buffer solution, mix and age for 24 hours, the lithium source is lithium nitrate; the iron The source is ferric nitrate; the phosphorus source is ammonium dihydrogen phosphate;
[0040] 2) Combine 95 parts by weight of the mixture obtained in step 1) with 3 parts by weight of thermoplastic starch, 1 part by weight of nitrogen-containing conjugated polymer polyvinyl carbazole, 0.5 parts by weight of control agent ammonium oxalate, 0.5 parts by weight High-quality processing lubrication aids, input into the vertical shaft reflective micro pulverizer, the rotor speed is controlled at 1200rpm, the strong stirring is for 5 minutes, the temperature is controlled at 80°C, through the dual pulverization function of blade cutting and high-speed airflow collision, during the blade cutting and pulverization process, the rotor Generate high-speed airflow to rot...
Embodiment 2
[0044] 1) Mix the lithium source, iron source, and phosphorus source in a molar ratio Li:Fe:P=1:1:1, add an appropriate amount of buffer solution, mix and age for 48 hours, the lithium source is lithium hydroxide; the The iron source is ferrous acetate; the phosphorus source is diammonium hydrogen phosphate;
[0045] 2) Mix 100 parts by weight of the mixture obtained in step 1) with 4 parts by weight of thermoplastic starch, 2 parts by weight of nitrogen-containing conjugated polymer polypyrrole, 1 part by weight of control agent magnesium chloride, and 0.5 parts by weight of processing lubricant The agent is input into the vertical shaft reflection micro pulverizer, the rotor speed is controlled at 1500rpm, the strong stirring is carried out for 10 minutes, and the temperature is controlled at 80°C. Through the double pulverization function of blade cutting and high-speed airflow collision, during the blade cutting and pulverization process, the rotor generates high-speed airf...
Embodiment 3
[0049] 1) Mix the lithium source, iron source, and phosphorus source in a molar ratio Li:Fe:P=1:1:1, add an appropriate amount of buffer solution, mix and age for 30 hours, the lithium source is lithium nitrate; the iron Source is ferric chloride; Described phosphorus source is diammonium hydrogen phosphate;
[0050] 2) Process 98 parts by weight of the mixture obtained in step 1) with 5 parts by weight of thermoplastic starch, 3 parts by weight of nitrogen-containing conjugated polymer polyaniline, 0.5 parts by weight of control agent silica, and 0.3 parts by weight Lubricating additives are input into the vertical shaft reflective micro pulverizer, the rotor speed is controlled at 1200rpm, the strong stirring is for 15 minutes, the temperature is controlled at 120°C, through the double pulverization function of blade cutting and high-speed airflow collision, during the blade cutting and pulverization process, the rotor generates high-speed The airflow rotates with the cuttin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Radial diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com