Fluorene-based hole transport compound
A technology of hole transport and compound, which is applied in the field of fluorene-based hole transport compounds, can solve the problems of high energy consumption for preparation, affect device efficiency, and poor thermal stability, and achieve high luminous purity, high luminous efficiency, and good thermal stability sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Synthesis of Compound 2
[0063]
[0064] Synthesis of Compound 2-1
[0065] In the reaction flask, add fluorenone (9g, 50mmol) and water (30ml), heat to 80°C, slowly add liquid bromine (8.8g, 55mmol) into the reaction flask, react for 4 hours, add 100ml of water and 100ml of 10% Aqueous solution of sodium bisulfate. After filtration, the obtained solid was recrystallized with absolute ethanol to obtain 11 g of product with a yield of 85%.
[0066] Synthesis of compound 2-2
[0067] In the reaction flask, add compound 2-1 (2.58g, 10mmol) and 15ml tetrahydrofuran, add dropwise phenylmagnesium bromide Grignard reagent (12mmol) dissolved in tetrahydrofuran at 0°C, and slowly rise to room temperature after the addition is completed. Reaction 12 After 1 hour, dilute hydrochloric acid was added to adjust the pH to 7, extracted with ethyl acetate, dried over anhydrous magnesium sulfate, and the solvent was removed. The crude product was chromatographed on a silica gel co...
Embodiment 2
[0076] Synthesis of compound 22
[0077]
[0078] In the reaction flask, add compound 2-3 (0.5g, 0.89mmol), 4-triphenylamine borate (0.3g, 1mmol), tetrahydrofuran (10ml), tetrakis (triphenylphosphine) palladium (50mg), potassium carbonate aqueous solution (2mol / l, 5mL), heated under reflux under the protection of nitrogen to react overnight. Stop the reaction, extract three times with dichloromethane, combine the organic phases, and wash with water until neutral; separate the organic phases, add anhydrous magnesium sulfate to dry, filter with suction, and spin dry; silica gel column chromatography gives 340 mg of white solids, yield 52 %.
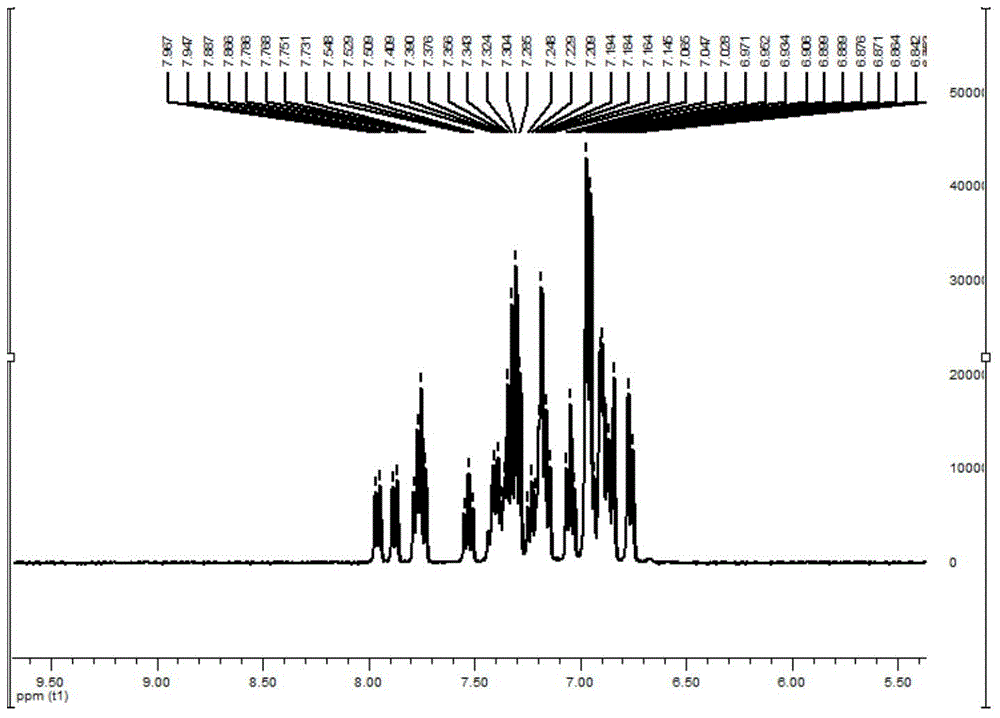
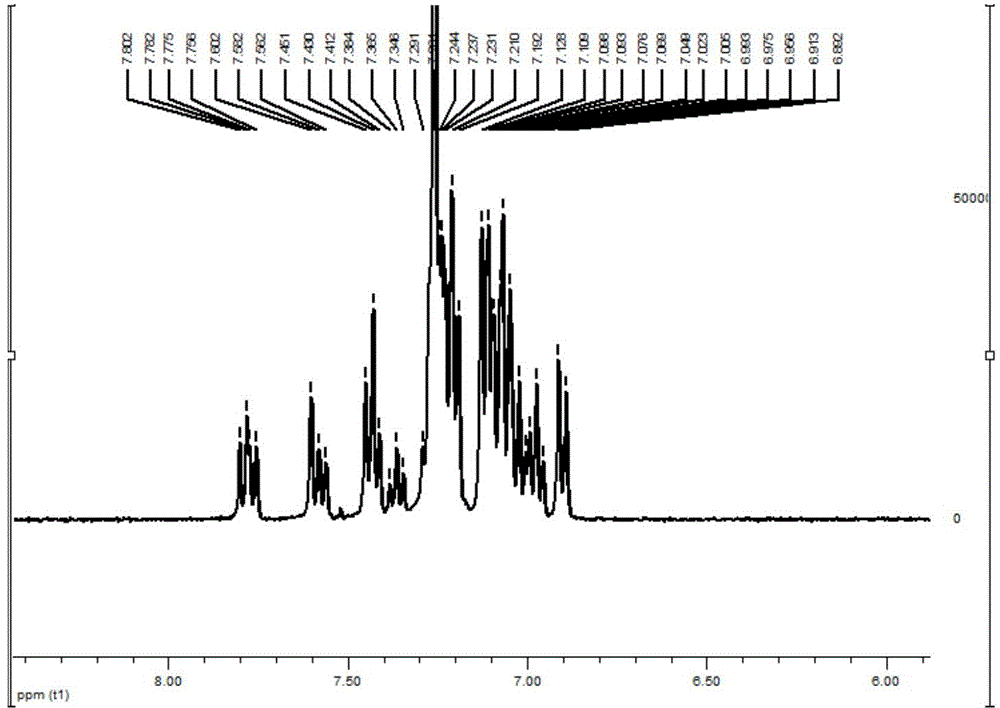
[0079] 1 H NMR (400MHz, CDCl 3 )δ7.76-7.80(m,2H),7.56-7.60(m,2H),7.35-7.45(m,4H),7.19-7.29(m,14H),6.89-7.13(m,18H).ESI, m / z: [M+H]+: 729.4. See NMR spectrum figure 2
Embodiment 3
[0081] Synthesis of compound 28
[0082]
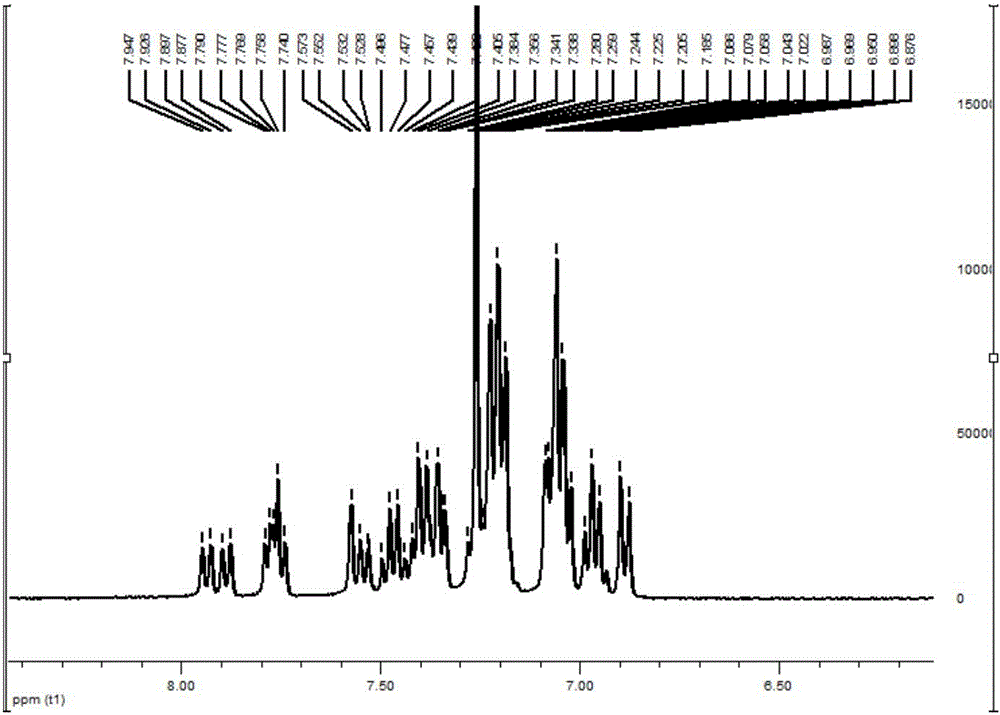
[0083] In the reaction flask, add compound 2-3 (0.2g, 0.35mmol), 4-(1-naphthylanilino) phenylboronic acid (0.15g, 0.44mmol), tetrahydrofuran (10ml), tetrakis (triphenylphosphine) Palladium (10 mg), potassium carbonate aqueous solution (2 mol / l, 5 mL), heated to reflux under nitrogen protection overnight. Stop the reaction, extract three times with dichloromethane, combine the organic phases, and wash with water until neutral; separate the organic phases, add anhydrous magnesium sulfate to dry, filter with suction, and spin dry; silica gel column chromatography gives 170 mg of white solids, yield 63 %. 1 H NMR (400MHz, CDCl 3 )δ7.88-7.95(m,2H),7.74-7.90(m,3H),7.34-7.57(m,11H),7.19-7.28(m,11H),6.88-7.09(m,15H).ESI, m / z: [M+H]+: 779.4. See NMR spectrum image 3
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com