Antigenic mimic epitope C of norfloxacin and application thereof
A mimetic epitope, norfloxacin technology, applied in the direction of material inspection products, instruments, analytical materials, etc., can solve the health and environmental threats of testing personnel, restrict the application and promotion of immunological detection methods, and norfloxacin is expensive Carcinogenicity and other issues, achieve good results, save costs, and reduce human health hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1. Affinity panning and identification of norfloxacin antigen mimotope
[0024] 1) Affinity panning of norfloxacin antigen mimic epitopes: the specific method is: dilute anti-norfloxacin monoclonal antibody with 10 mM PBS (pH 7.4), and coat 96 wells with a final concentration of 100 μg / mL ELISA plates were incubated overnight at 4°C. The next day, after washing 10 times with TBST (50 mM NaCl, pH 7.5 containing 0.1% Tween-20 (v / v) ), add 300 μl of blocking solution (3% BSA-PBS) and incubate at 4°C for 2 hours. After 2 hours, discard the blocking solution, wash 5 times with TBST, add 100 μl phage peptide library (phage display ring heptapeptide library or dodecapeptide library, purchased from NEB Company, dilute the phage stock solution 10 times with TBS, about 1.0× 10 11 pfu), shake and react for 1 hour at 22-26°C. Unbound phages were discarded, washed 10 times with TBST, bound phages were eluted with 0.2 M Glycine-HCl (pH 2.2), and immediately neutralized wi...
Embodiment 2
[0028] Example 2. The sequencing of the mimotope encoding gene of norfloxacin antigen and the determination of its amino acid sequence
[0029] The phages identified by indirect competition ELISA displaying mimotopes of the norfloxacin antigen were amplified, and the DNA sequencing templates of the phages were extracted. The brief process is as follows: carry out phage amplification, after the first step of centrifugation, transfer 800 μl of phage-containing supernatant into a new centrifuge tube. Add 200 μl PEG / NaCl to precipitate the phage. After centrifugation, resuspend the pellet in 100 μl iodide buffer (10 mM Tris-HCl (pH 8.0), 1 mM EDTA, 4 M NaI), add 250 μl absolute ethanol to precipitate DNA, centrifuge and wash with 70% ethanol Precipitation (DNA sequencing template). The pellet was finally resuspended in 20 μl of sterilized water, and 2 μl was taken for agarose gel electrophoresis analysis; 5 μl of phage template was used for DNA sequencing, and its -96 gIII seque...
Embodiment 3
[0030] Example 3. The application of mimotopes of norfloxacin antigens as competitive antigens in ELISA
[0031] (1) Sample extraction
[0032] Weigh 5g of the sample (cereals and related foods), add 25ml of 60% methanol-PBS solution, shake at 200 rpm for 5 minutes; filter the extract with No. 1 filter paper, take 1ml of the filtrate and add 4ml of PBS ( Phosphate buffer, pH=7.2)
[0033] After mixing, the sample extract is ready for use.
[0034] (2) Coating and sealing
[0035] Dilute the anti-norfloxacin monoclonal antibody with 10 mM PBS (pH 7.4), coat the microtiter plate with 10 μg / mL, and incubate overnight at 4°C. The next day, after washing 3 times with PBST (10 mM PBS, 0.05% Tween-20 (v / v)), block with PBS containing 3% skimmed milk powder, incubate at 37°C for 1 hour, then wash the plate 6 times with PBST stand-by.
[0036] (3) Establishment of standard curve
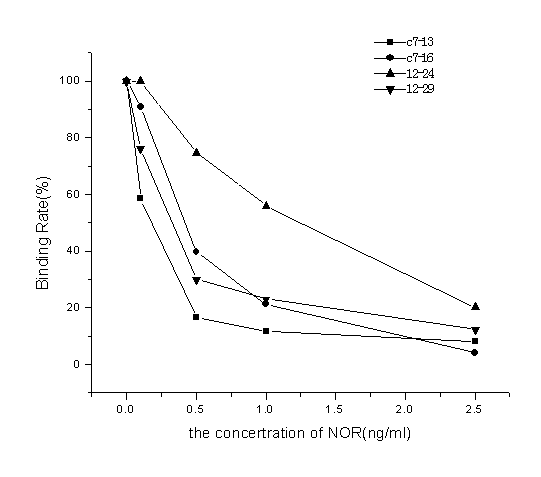
[0037] Take out the plates treated in step (2), and put 50 μl of phage displaying mimotopes of norfl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com