Structure, preparation and biological activity of A type procyanidine biopolymer and acetone condensation compound
A technology of acetone condensate and proanthocyanidin, applied in the field of chemistry, can solve the problems of complex changes in structure, high irritation and toxicity, etc., and achieve the effects of strong free radical scavenging activity, low irritation and toxicity, and increased lipophilicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0014] The following specific embodiments will further describe the preparation method and activity assay method of the present invention in detail.
[0015] A preparation method and structure of proanthocyanidin acetone condensate, comprising the following steps:
[0016] 1) Preparation of Proanthocyanidin Acetone Condensate
[0017] Dissolve proanthocyanidins in acetone. After the mixture was cooled in ice water, a catalytic amount of concentrated sulfuric acid was added dropwise, and after reacting at room temperature, sodium carbonate was added to neutralize.
[0018] 2) Purification of Proanthocyanidin Acetone Condensate
[0019] The above reaction solution was diluted with ice water and subjected to reverse phase silica gel C18 chromatographic separation with water-methanol gradient elution to obtain proanthocyanidin acetone condensate.
Embodiment 1
[0020] Embodiment 1: the preparation of catechin acetone condensate
[0021] Catechin (60 mg, 0.2 mmol) was dissolved in acetone (1.4 ml). The mixture was cooled in ice water, and concentrated sulfuric acid (0.012 ml) was added dropwise. React at room temperature for 70 min. The reaction solution was neutralized to pH=5-6 by adding sodium carbonate (0.12 g). Add 20-fold dilution with ice water, use reversed-phase silica gel C18 column separation, and obtain 43 mg of compound from the elution fraction of 30% methanol 1 (Yield 65%). compound 1 The NMR spectrum data are consistent with the planar derivatives of catechin reported in the literature.
Embodiment 2
[0022] Embodiment 2: the preparation of proanthocyanidin A1 acetone condensate
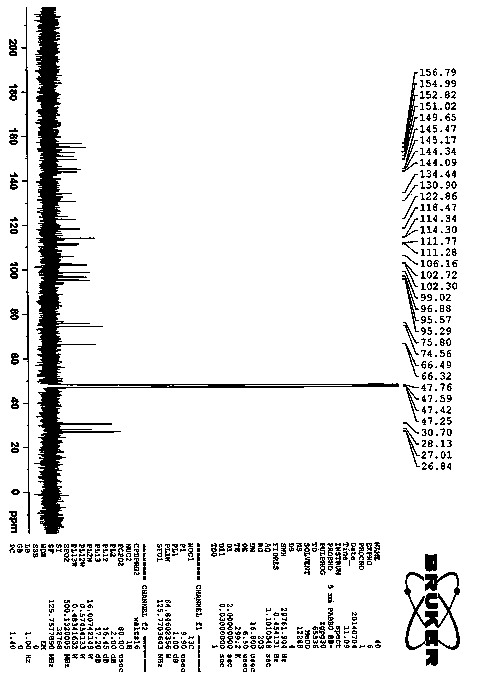
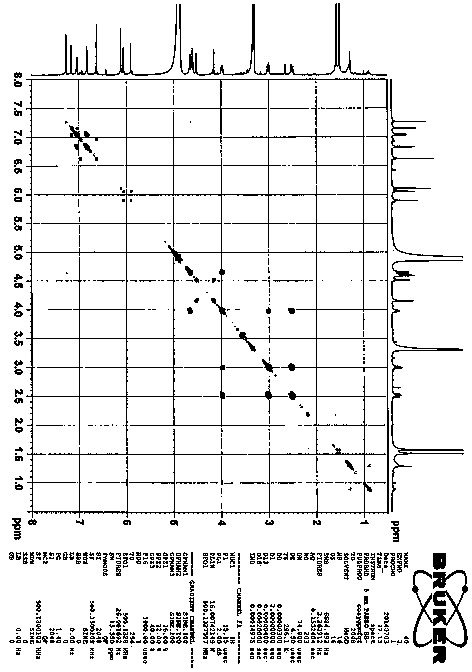
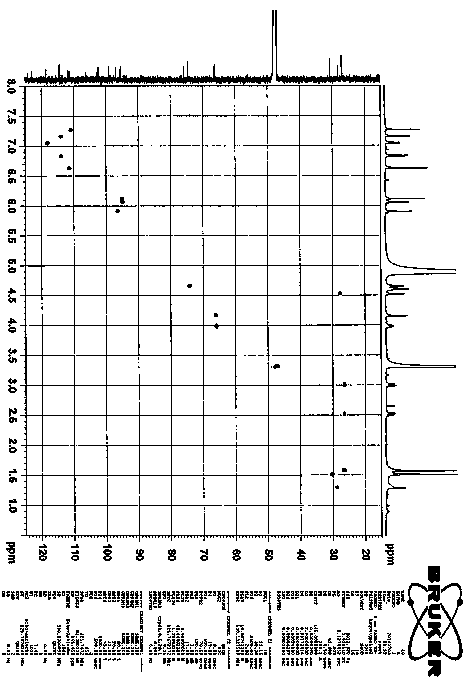
[0023] Procyanidin A1 (95 mg, 0.16 mmol) (Zhang H, Yerigui, Yang Y, Ma C. Structures and antioxidant and intestinal disaccharidase inhibitory activities of A-typeproanthocyanidins from peanut skin. J Agric Food Chem , 2013, 61: 8814-8820) was dissolved in acetone (1.75 ml), the mixture was cooled in ice water, and concentrated sulfuric acid (0.012 ml) was added dropwise. React at room temperature for 70 min. The reaction solution was neutralized to pH=5-6 by adding sodium carbonate (0.12 g). Add 20 times of ice water to dilute, use reverse phase silica gel C18 chromatography column to separate, obtain 30.9 mg compound 2 , yield 33% ( Attachment 1 ). This compound is a new compound that has not been reported in the literature. Through comprehensive analysis of various spectral data ( Figure 3-7 ) determines the structure. compound 2 of 1 H and 13 CNMR data such as attached Figure 9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com