High-capacity lithium-enriched positive electrode material and preparation method thereof
A lithium-rich positive electrode material and high-capacity technology, applied in the field of electrochemistry, can solve the problems of material structure collapse, lithium ion diffusion rate reduction, and material cycle stability deterioration, so as to reduce capacity loss, improve cycle stability and high The effect of low rate performance and electrochemical impedance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
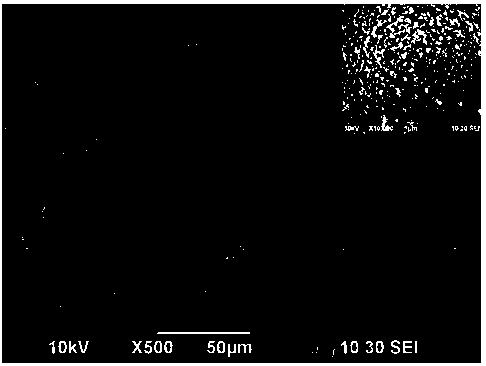
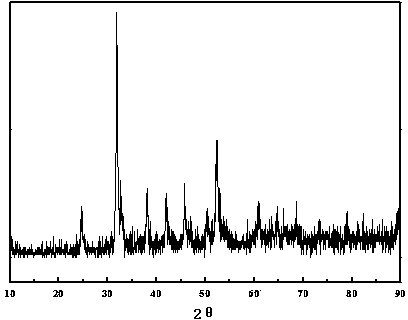
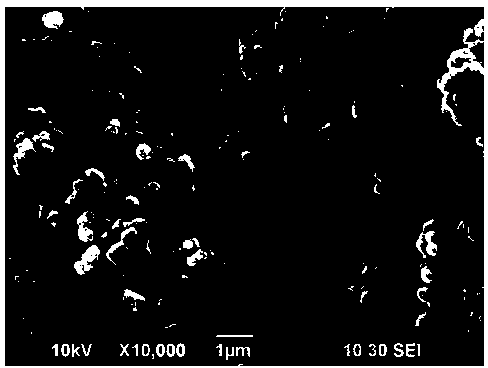
[0047] The molar ratio is (0.54:0.13:0.13) to prepare manganese acetate, cobalt acetate, nickel acetate mixed solution, the total concentration of transition metal ions is 0.08mol / L, the concentration of prepared urea solution is 0.16mol / L, and the volume ratio is 1: 1 After mixing the two solutions for half an hour, transfer to a 100ml reaction kettle (50% fill). The reaction was carried out at 90°C for 3h, at 110°C for 3.5h, and finally at 170°C for 15h. Naturally cool to room temperature, suction filter, wash until the pH is about neutral, and dry at 50°C for 24 hours to obtain a carbonate precursor. It looks like figure 1 , the particles are spherical and uneven in size. From the enlarged picture of a single particle, it can be seen that spherical particles are aggregates formed by many nanoparticles, which are mesogens assembled and synthesized in situ by different components, with larger specific surface area and high porosity. Its XRD is figure 2 , with MnCO 3 The...
Embodiment 2
[0050] According to the molar ratio (0.52:0.13:0.13:0.02), the mixed solution of manganese sulfate, cobalt sulfate, nickel sulfate and yttrium sulfate is prepared, the total concentration of transition metal ions is 0.08mol / L, and the concentration of the prepared urea solution is 0.32mol / L. The ratio was 1:1. After mixing the two solutions for half an hour, they were transferred to a 100ml reactor (50% fill). The reaction was carried out at 90 °C for 3 h, at 110 °C for 3.5 h, and finally at 190 °C for 15 h. Naturally cooled to room temperature, suction filtered, washed until the pH was about neutral, and dried at 50 °C for 24 h to obtain a carbonate precursor. It looks like Figure 6 As shown, it is a spherical particle, a mesogenic material composed of many small particles.
[0051] According to the stoichiometric ratio = 1.2:0.8, lithium carbonate and the precursor were weighed respectively, and absolute ethanol was used as a dispersant, and the water bath was placed at 6...
Embodiment 3
[0053] Prepare a mixed solution of manganese nitrate, cobalt nitrate, nickel nitrate and yttrium nitrate in molar ratio (0.52:0.13:0.13:0.02), the total concentration of transition metal ions is 0.16mol / L, and the concentration of prepared urea solution is 0.40mol / L. The ratio was 1:1. After mixing the two solutions for half an hour, they were transferred to a 100ml reactor (50% fill). The reaction was carried out at 90 °C for 3 h, at 110 °C for 3.5 h, and finally at 210 °C for 15 h. Naturally cooled to room temperature, suction filtered, washed until the pH was about neutral, and dried at 50 °C for 24 h to obtain a carbonate precursor. It looks like Figure 8 As shown, the spherical particles are a mesogenic material composed of many small particles.
[0054] According to the stoichiometric ratio of 1.15:0.8:0.05, lithium hydroxide, precursor and sodium hydroxide were weighed, and absolute ethanol was used as a dispersant, and the water bath was placed at 60 °C until the ab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com