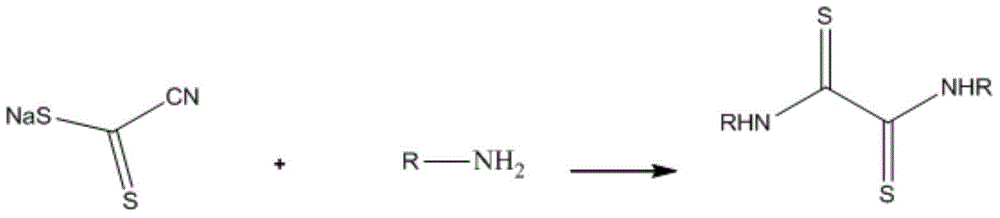
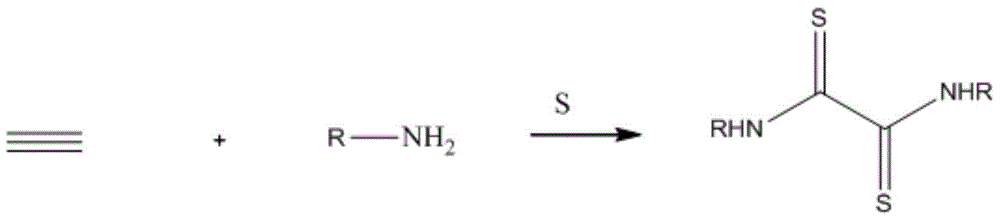N,N'-dialkyl dithiooxamide as well as preparation method and application thereof
A technology of dialkyldithiooxamide and oxamide, applied in the N field, can solve the problems of cumbersome operation, complicated operation, expensive raw material and the like, and achieves low raw material and manufacturing cost, high reaction yield and low price. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation of N, N'-di-n-butyldithiooxamide
[0043] The first preparation method of N,N'-di-n-butyl oxamide is to add n-butylamine 40g, pyridine 44.3 g and toluene 400mL, after stirring evenly, cool to 0-5°C with an ice-salt bath, and slowly add 33g of oxalyl chloride dropwise. Then reacted at room temperature for 4 hours, filtered to obtain a light orange solid, washed twice with dilute hydrochloric acid, dried after washing with water, concentrated and filtered to obtain 39 g of white crystal N, N'-di-n-butyl oxalamide, yield 75%, melting point 157 ~158°C;
[0044] Preparation method 2 of N,N'-di-n-butyl oxalamide: Add 500mL of toluene and 153g of n-butylamine into the reaction bottle, slowly add 140g of diethyl oxalate under stirring, heat up to reflux, the reaction produces ethanol, and the reflux temperature is 90 ℃, reflux reaction for 3 hours, the reflux device is a distillation device, slowly distill out the by-product ethanol to make the reaction complete, ...
Embodiment 2
[0048] Embodiment 2: the preparation of N, N'-didodecyl dithiooxamide
[0049] N, N'-didodecyl oxalamide preparation method 1, add n-dodecylamine 190g in the three-necked bottle equipped with a stirrer, a reflux condenser with a drying tube and an exhaust gas absorption device, and a dropping funnel, three 104g of ethylamine and 1000mL of toluene were stirred evenly, cooled to 0-5°C with an ice-salt bath, and 63.5g of oxalyl chloride was slowly added dropwise. Then reacted at room temperature for 4 hours, filtered to obtain a light orange solid, washed twice with dilute hydrochloric acid, dried after washing with water, concentrated and filtered to obtain 180.5 g of white crystal N, N'-didodecyl oxalamide, yield 85%, Melting point 123~124℃;
[0050]Preparation method 2 of N, N'-didodecyl oxalamide: Add 1000 mL of toluene and 190 g of n-dodecylamine into the reaction flask, slowly add 59 g of dimethyl oxalate under stirring, heat up to reflux, the reaction produces methanol, a...
Embodiment 3
[0053] Embodiment 3: the preparation of N, N'-dibenzyl dithiooxamide
[0054] N, N'-dibenzyl oxamide preparation method 1, add benzylamine 110g, triethylamine 103.7g in the three-neck bottle equipped with agitator, reflux condenser with drying tube and tail gas absorption device, dropping funnel and 550mL of toluene, stirred evenly, cooled to 0-5°C with an ice-salt bath, and slowly added 63.5g of oxalyl chloride dropwise. Then reacted at room temperature for 4 hours, filtered to obtain a light orange solid, washed twice with dilute hydrochloric acid, dried after washing with water, concentrated and filtered to obtain 111.3 g of white crystal N, N'-dibenzyl oxalamide, yield 83%, melting point 224 ~225°C;
[0055] Preparation method 2 of N,N'-dibenzyloxalamide: Add 550mL of toluene and 110g of benzylamine into the reaction flask, slowly add 59g of dimethyl oxalate under stirring, heat up to reflux, the reaction produces methanol, and the reflux temperature is about 90°C. Reflu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com