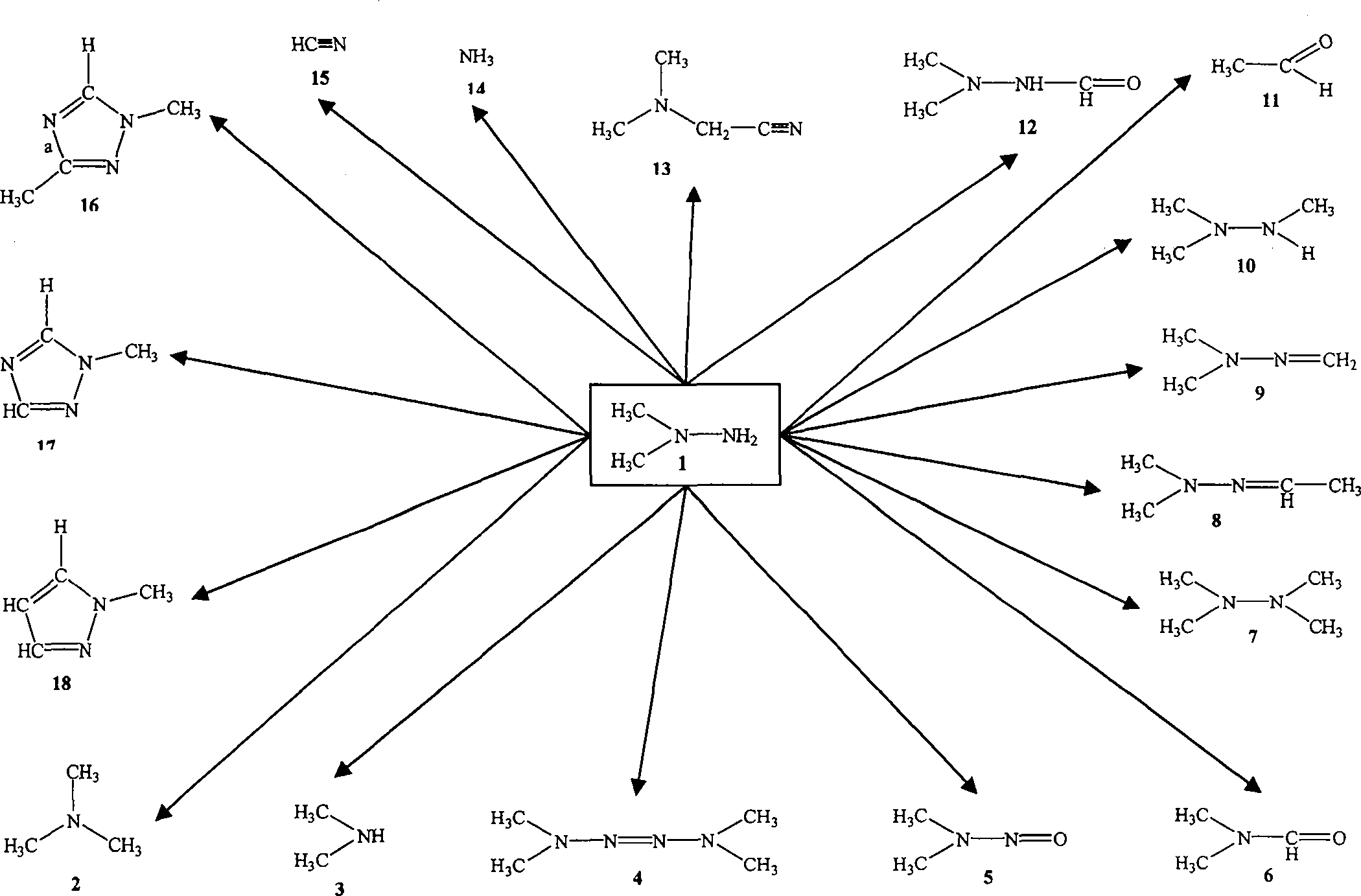
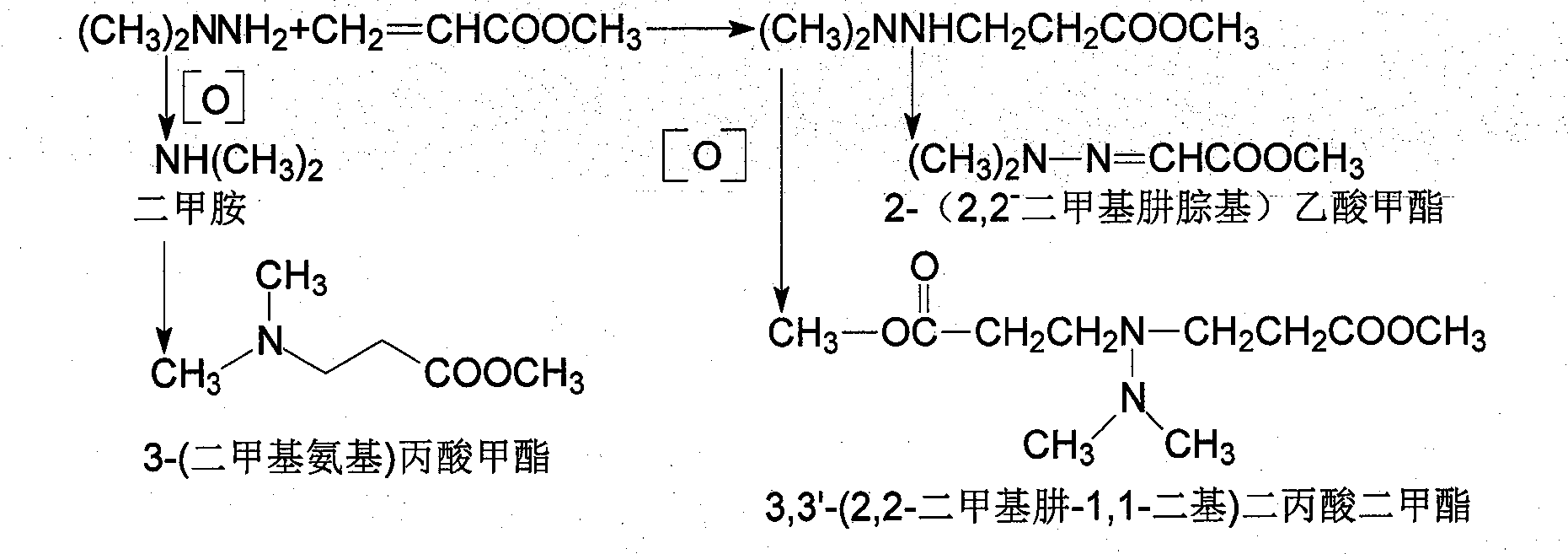
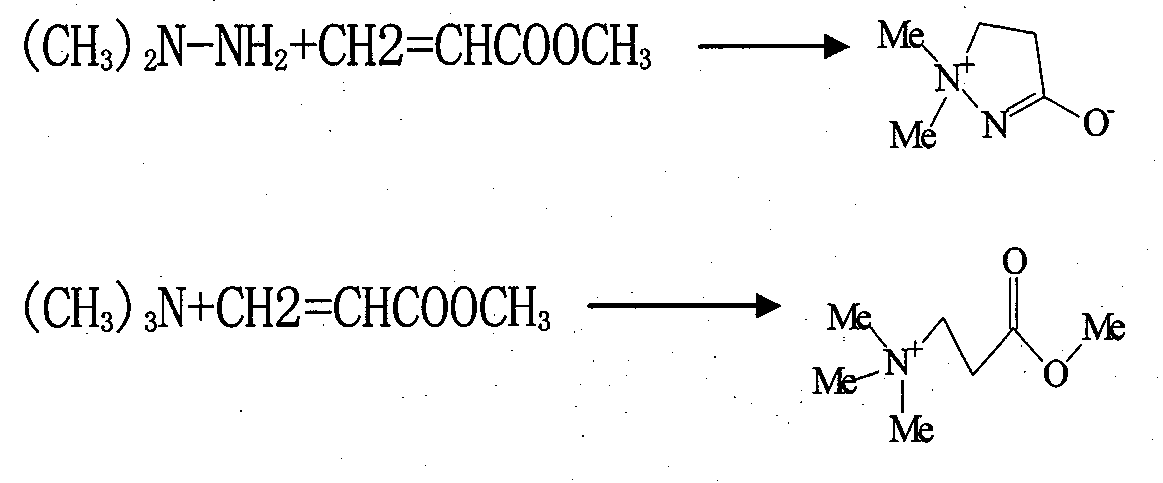Method for improving preparation technology of 3-(2,2-dimethylhydrazino)methyl propionate and 3-(2,2,2-trimethylhydrazino)methyl propionate
A technology of dimethylhydrazino and methyl propionate, which is applied in the field of preparation of methyl 3-propionate and methyl 3-propionate salt, can solve the problem of reaction temperature and pressure to accelerate alkylation reaction, easy oxidation, Increase production costs and other issues to achieve the effect of ensuring product quality, reducing post-processing difficulties, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Add methyl acrylate 172g (2mol) in the three-neck round-bottomed flask with mechanical stirrer, dropping funnel and reflux condenser, under cooling, dropwise the 1,1-dimethylhydrazine of 132g (2.2mol), After 2 hours the dropwise was completed, then the reaction mixture was heated at the same temperature until methyl 3-(2,2-dimethylhydrazino)propionate was formed (monitored by GM-MS).
[0046] Dissolve the above-mentioned 3-(2,2-dimethylhydrazino)propionate methyl ester product in 40% ethanol (350ml), cool to 0°C, pass through methyl bromide (189g, 1.99mol) for 1 hour, and continue at the same temperature Stir for 0 to 5 hours, cool and filter the yellowish or brown precipitate, and wash the crude product with acetone (1000ml). Dry to obtain yellow 3-(2,2,2-trimethylhydrazine) methyl propionate bromide crude product 265.7g, yield 91.0%,; Crude product is recrystallized with ethanol, dry to obtain light yellow crystalline powder 219.8g, product The yield is 75.3%, the me...
Embodiment 2
[0048] Repeat the method in the above-mentioned Example 1 and dry to obtain brown yellow 3-(2,2,2-trimethylhydrazine) methyl propionate bromide crude product 261.3g, yield 89.5%; Crude product is recrystallized with ethanol, dried to obtain White crystalline powder 214.2g, yield 73.4%, melting point 133-135 ° C, main content 102.5% (verified by stability experiments, the stability is poor, and the color gradually becomes darker, because the contained trace impurities have a greater impact on the product, It was found that the molecular formula decayed, and various indicators changed greatly, and the product was valid for a maximum of two years).
Embodiment 3
[0050] Repeat the method in the above-mentioned Example 1 and dry to obtain light yellow 3-(2,2,2-trimethylhydrazine) methyl propionate bromide crude product 271.5g, yield 89.5%; The crude product is recrystallized with ethanol, dried to obtain White crystalline powder 225.3g, yield 77.2%, melting point 132-134°C, main content 103.0% (verified by stability experiments, the stability is poor, and the color gradually becomes darker, because the contained trace impurities have a greater impact on the product, It was found that the molecular formula decayed, and various indicators changed greatly, and the product was valid for a maximum of two years).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com