Difunctional negative electrode and its application as all-vanadium redox energy storage battery negative electrode
An all-vanadium flow, energy storage battery technology, applied in battery electrodes, fuel cells, circuits, etc., can solve problems such as being unsuitable for large-scale application, high electrode cost, reducing hydrogen evolution, etc., to improve electrocatalytic activity and electrochemical performance. Reversibility, simple preparation method, and the effect of inhibiting hydrogen evolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] A certain size of carbon felt was impregnated in 0.01M Bi(NO 3 ) 3 HNO 3 solution, after ultrasonic dispersion for 30 min, take it out, put it in a drying oven at 105 ° C for 10 h, and then place the loaded Bi(NO 3 ) 3 The carbon felt was heated to 600°C in a nitrogen atmosphere, and H 2 Constant temperature reaction 1h, the Bi 3+ It was reduced to Bi, then cooled to room temperature under a nitrogen atmosphere, and weighed using an electronic balance to determine that the mass ratio of Bi loading was 1%.
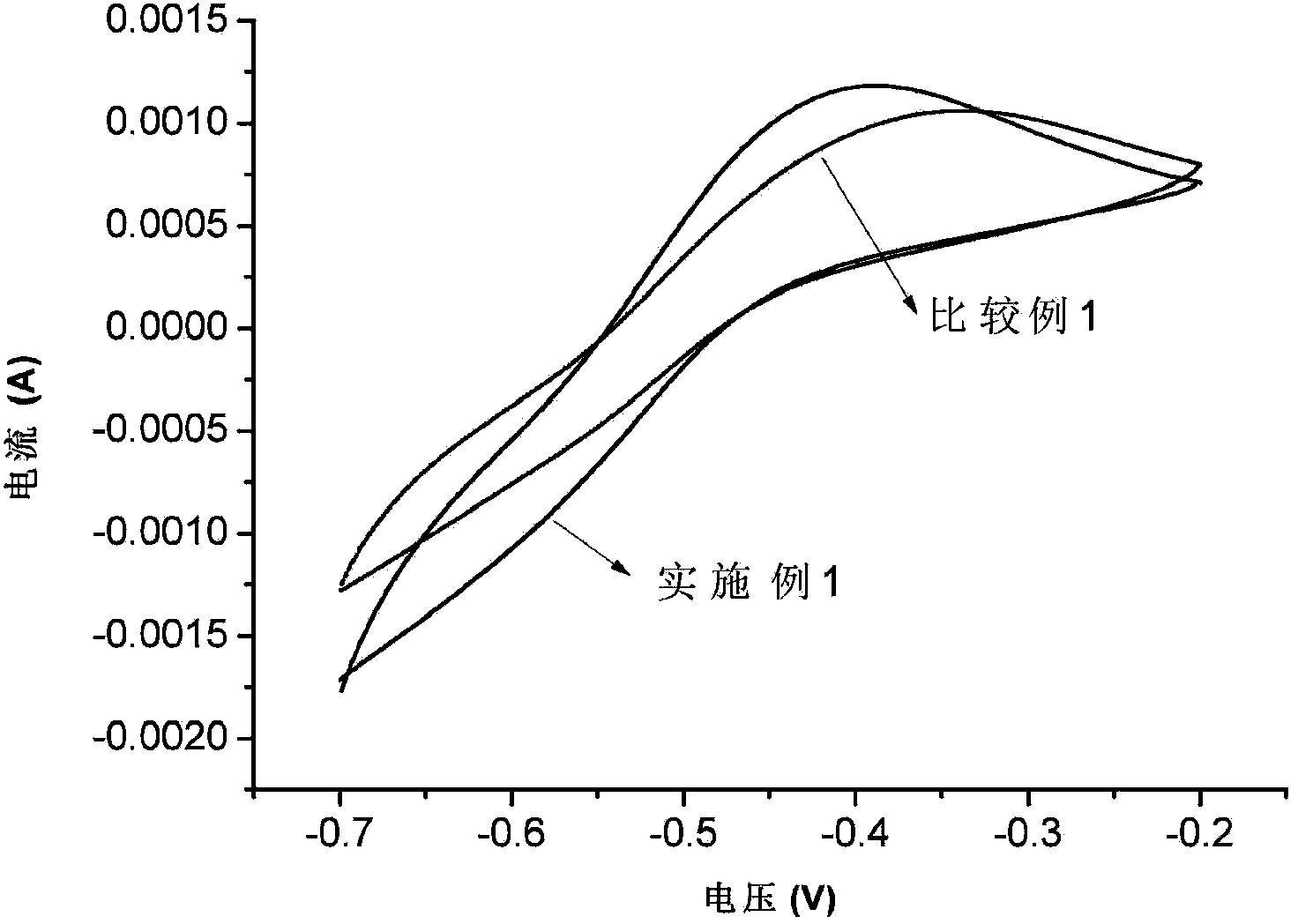
[0054] In order to test the electrochemical activity of vanadium ion redox couple on the surface of Bi-modified carbon felt, the Bi-modified carbon felt prepared in Example 1 was tested by cyclic voltammetry. Bi-modified carbon felt was used as the working electrode, a non-porous graphite plate was used as the counter electrode, and a saturated calomel electrode was used as the reference electrode. The electrochemical testing instrument used was the CHI612 elect...
Embodiment 2
[0065] The electrodeposition solution consists of 12g / L BiCl 3 , 55g / L tartaric acid, 100g / L glycerin and 45g / L sodium chloride solution, the pH value of the solution is adjusted to about 1.0 with dilute hydrochloric acid. A carbon felt of a certain size is used as the working electrode, and the counter electrode is a graphite plate. Direct current electrochemical deposition is adopted, and the current density is 10mA / cm 2 , the deposition time is 10s. The mass ratio of Bi loading was determined to be 1% by weighing with an electronic balance.
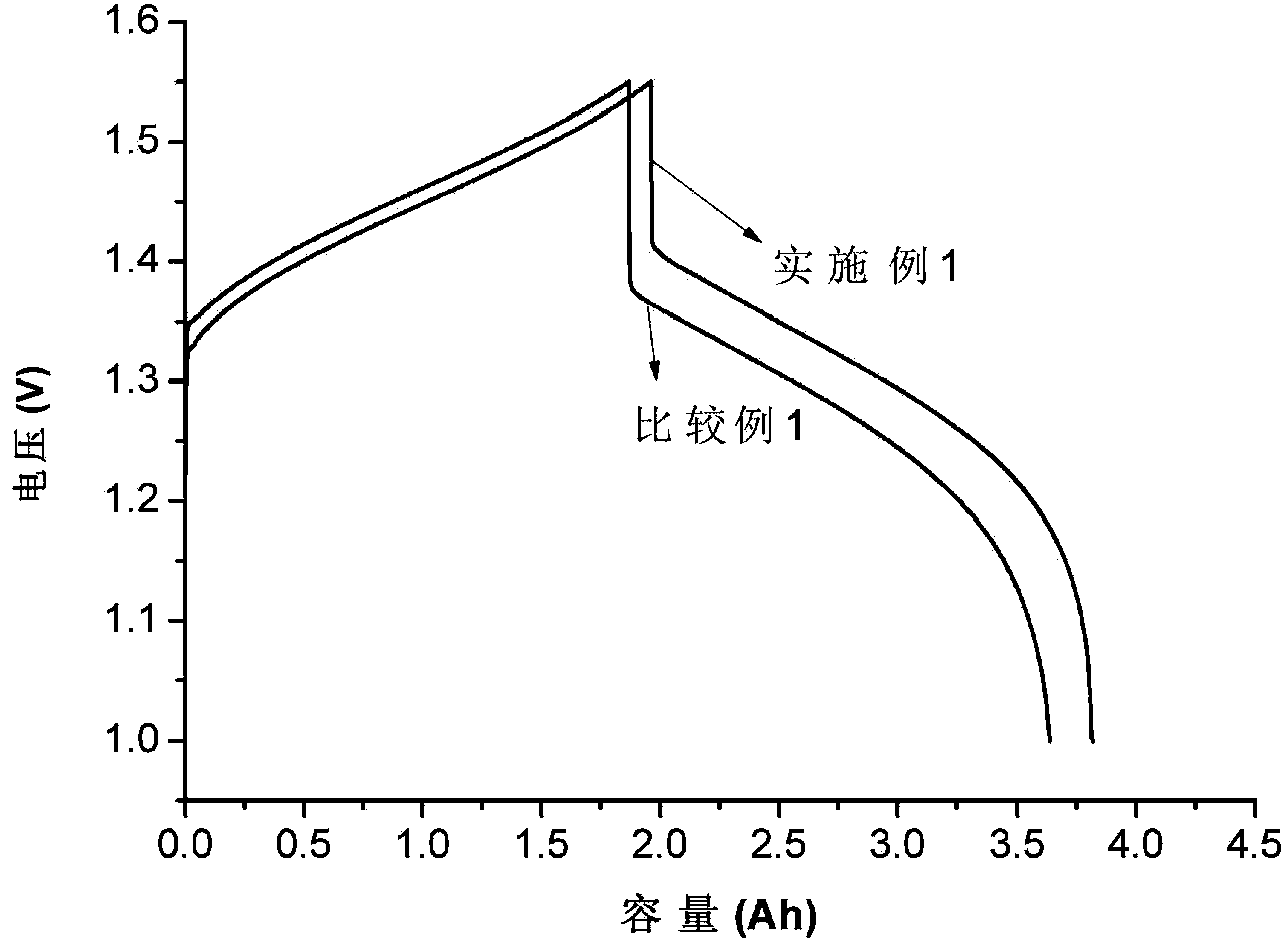
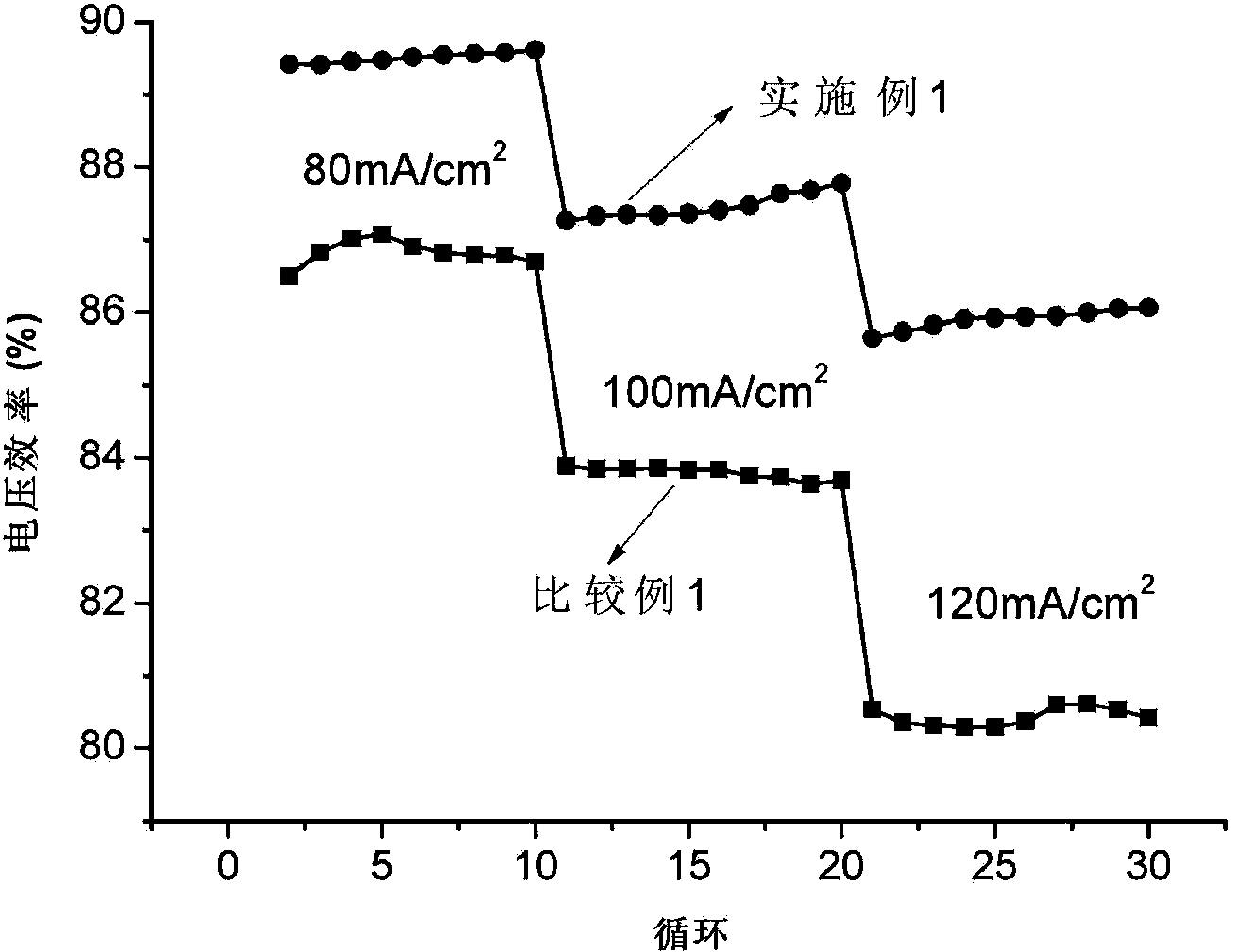
[0066] The single cell assembly evaluation conditions are the same as in Example 1, and the difference from Example 1 is that the all-vanadium redox flow battery using the Bi-modified carbon felt in this example as the negative electrode has a current density of 80mA / cm 2 , the voltage efficiency and energy efficiency were 89.3% and 84.2% respectively; the current density increased to 120mA / cm 2 , the voltage efficiency and energy e...
Embodiment 3
[0068] A certain size of graphite felt was impregnated in 0.02M Bi(NO 3 ) 3 in the ethylene glycol solution, ultrasonically dispersed for 30min, took it out, put it in a drying oven at 200°C for 10h, and then loaded the Bi(NO 3 ) 3 The graphite felt is heated up to 500°C in a nitrogen atmosphere, and H 2 Constant temperature reaction 2h, Bi 3+ It was reduced to Bi, then cooled to room temperature under a nitrogen atmosphere, and weighed using an electronic balance to determine that the mass ratio of Bi loading was 2%.
[0069] The single cell assembly evaluation conditions are the same as in Example 1, except that the difference from Example 1 is that the current density of the all-vanadium redox flow battery using Bi-modified graphite felt as the negative electrode in this example is 80mA / cm 2 , the voltage efficiency and energy efficiency were 88.9% and 84.2% respectively; the current density increased to 120mA / cm 2 , the voltage efficiency and energy efficiency remaine...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com