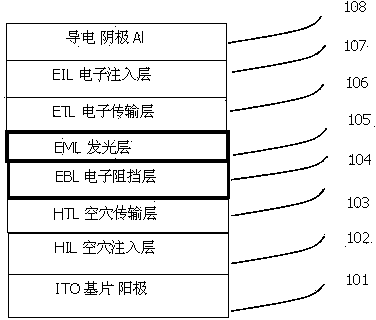
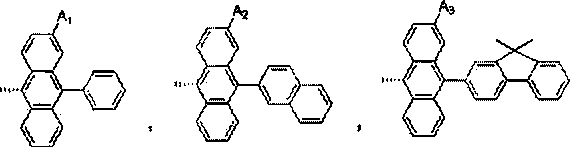
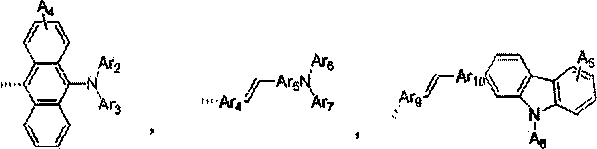
Substituted benzophenanthrene derivative organic light emitting diode material
A light-emitting diode and organic technology, applied in the field of organic light-emitting diode materials that replace triphenylene derivatives, can solve problems such as degradation, high crystallinity, and poor thermal stability, and achieve the effects of improved efficiency and high thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0123] Example 1. Synthesis of Compound 42 (M5)
[0124] .
[0125] ( 1 ) Intermediate M1 Synthesis
[0126] In a 250 mL three-necked flask with a thermometer and a condenser, add 72 g (250 mmol) of 3-bromophenanthrenequinone, 58 g (276 mmol) of dibenzyl ketone, 7 g (125 mmol) of KOH, and 800 ml of ethanol, and heat to reflux. Reaction for 1h. The TLC dot plate monitors the reaction to completion. Cool to room temperature, filter, wash the filter residue with methanol, and filter to obtain the desired product. 58g (42%) of brown solid is received; it is directly used in the next reaction without purification.
[0127] ( 2 ) Intermediate M2 Synthesis
[0128] In a 1000 mL three-necked flask with a thermometer and a condenser, 58 g (125.5 mmol) of M1, 24.6 g (200 mmol) of trimethylsilyl acetylene, and 350 ml of o-xylene were sequentially added, and the temperature was raised to reflux for 12 hours. Cooled to room temperature, filtered, the filtrate was reversed-phase precipitati...
Embodiment 2
[0135] Example 2. Synthesis of compound 10
[0136] .
[0137] ( 1 ) Intermediate 3- Boron ester -5,8- Synthesis of diphenyltriphenylene
[0138] In a 100 mL three-necked flask with a thermometer and a condenser, add 0.92g (2mmol) of 3-bromo-5,8-diphenyltriphenylene, Pinacol diborate 0.61g (2.4 mmol), tribenzylidene acetone dipalladium 0.04g (0.04 mmol), S-phos 0.03g (0.08mmol), potassium acetate 0.4g (4 mmol), toluene 20ml, replaced with nitrogen, heated to reflux for reaction 16h. Cool to room temperature, filter, collect the filtrate, use a short silica gel column, use pure n-hexane as mobile phase: dichloromethane=4:1, and receive 0.68g (68%) of white solid, MS=612.
[0139] ( 2 ) Synthesis of the final product
[0140] In a 100 mL three-necked flask with a thermometer and a condenser, add 0.6 g (1.2 mmol) of 3-boron ester-5,8-diphenyltriphenylene, 9- bromine -10-(2- Naphthyl ) Anthracene 0.383g (1 mmol), Pd(PPh 3 ) 4 0.02g (0.016 mmol), potassium carbonate 0.175g (1...
Embodiment 3
[0141] Example 3. Synthesis of compound 52 (M7)
[0142] .
[0143] ( 1 ) Intermediate M6 Synthesis
[0144] In a 100 mL three-necked flask with a thermometer and a condenser, 22.8g (109mmol) of 2-amino-9,9-dimethylfluorene, 19.2g (72.87mmol) of 2-bromodibenzofuran, Sanya 1 g (1.09 mmol) of dipalladium benzylacetone, 1.35 g (21.8 mmol) of BINAP, 13.9 g (145 mmol) of sodium tert-butoxide, 180 ml of toluene, replaced with nitrogen, heated to reflux and reacted for 3h. Cool to room temperature, filter, collect the filtrate, use a short silica gel column, use pure n-hexane: dichloromethane = 4:1 as the mobile phase, and receive 23.2 g (85%) of white solid.
[0145] ( 2 ) Synthesis of the final product
[0146] In a 100 mL three-necked flask with a thermometer and a condenser, add 0.45 g (1.2 mmol) of M6, 0.458 g (1 mmol) of M3, 0.048 g (0.05 mmol) of tribenzylidene acetone, and P(t- Bu) 3 0.16g (0.2mmol), 0.38g (4mmol) of sodium tert-butoxide, 25ml of toluene, replaced with nitrog...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com