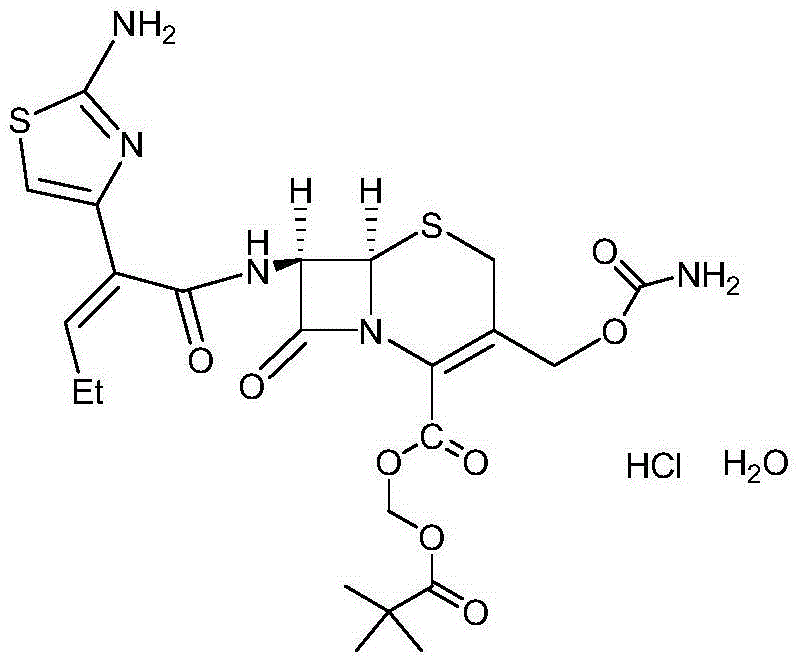
Refining method for cefcapene pivoxil hydrochloride
A technology of cefcapine hydrochloride and a refining method, applied in the direction of organic chemistry and the like, can solve the problems of poor stability, increased impurities in the finished product, influence on the quality of the finished product, etc., and achieves the effects of mild reaction conditions, reduced production costs, and environmental friendliness.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] In a 250ml four-neck bottle, add 9.4g of cefcapene axetil hydrochloride crude product, 50ml of methanol, and 100ml of chloroform, stir at room temperature, add 5% sodium hydroxide solution to adjust the pH to neutral, let stand to separate layers, and take the organic layer through Concentrate under reduced pressure to recover the organic solvent, add 100ml of methyl acetate to the residue, control the temperature at 10°C, add 17.5ml of pre-prepared 5% hydrochloric acid solution dropwise, keep stirring for 4 hours, cool to 0°C, filter, and use cold methyl acetate The ester was washed and dried to obtain the fine product of cefcapene hydrochloride. The product is in the form of white powder, the product purity is 99.8%, and the weight yield is 82%.
Embodiment 2
[0023] In a 250ml four-necked bottle, add 9.4g of crude product of cefcapene axetil hydrochloride, 50ml of isopropanol, and 100ml of dichloromethane, stir at room temperature, add 5% sodium bicarbonate solution to adjust the pH to neutral, let stand for stratification, and take the organic The layer was concentrated under reduced pressure to recover the organic solvent, and the residue was added with 100ml of ethyl acetate, the temperature was controlled at 15°C, 8.7ml of a pre-prepared 10% hydrochloric acid solution was added dropwise, kept stirring for 5 hours, cooled to 10°C, filtered, cold acetic acid Wash with ethyl ester and dry to obtain the fine product of cefcapene hydrochloride. The product is white powder, the product purity is 99.6%, and the weight yield is 79%.
Embodiment 3
[0025] In a 250ml four-necked bottle, add 9.4g of cefcapene axetil hydrochloride crude product, 75ml of acetone, and 100ml of chloroform, stir at room temperature, add 5% potassium carbonate solution to adjust the pH to neutral, let stand to separate layers, take the organic layer and reduce Concentrate under pressure to recover the organic solvent, add 100ml of butyl acetate to the residue, control the temperature at 20°C, add 8.7ml of pre-prepared 10% hydrochloric acid solution dropwise, keep stirring for 6 hours, cool down to 5°C, filter, and wash with cold butyl acetate , dried to obtain cefcapene hydrochloride white powder, product purity 99.5%, weight yield 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com