Large Stokes shift and near infrared fluorescence emitting new rhodamine fluorescent dye and synthetic method thereof
A technology of Stokes and fluorescent dyes, which is applied in the field of new rhodamine-based fluorescent dyes and their synthesis, which can solve the problems of complex dye synthesis, staying in the stage of small amount of preparation in the laboratory, and small Stokes shift, and achieve the goal of synthesis The effect of simple steps, cheap raw materials, and easy products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
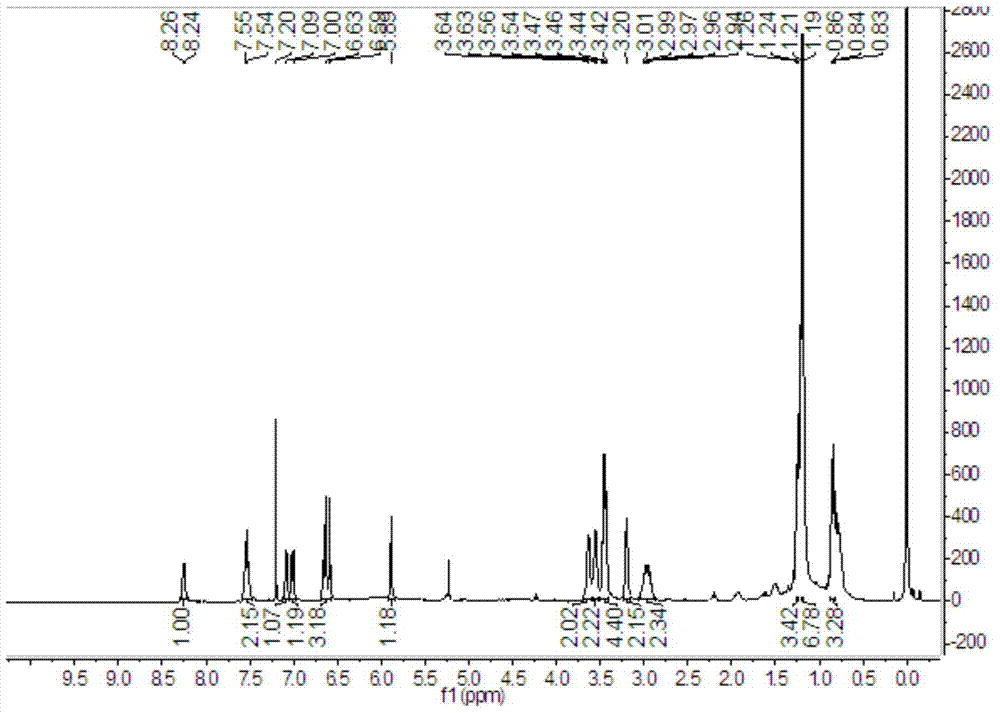
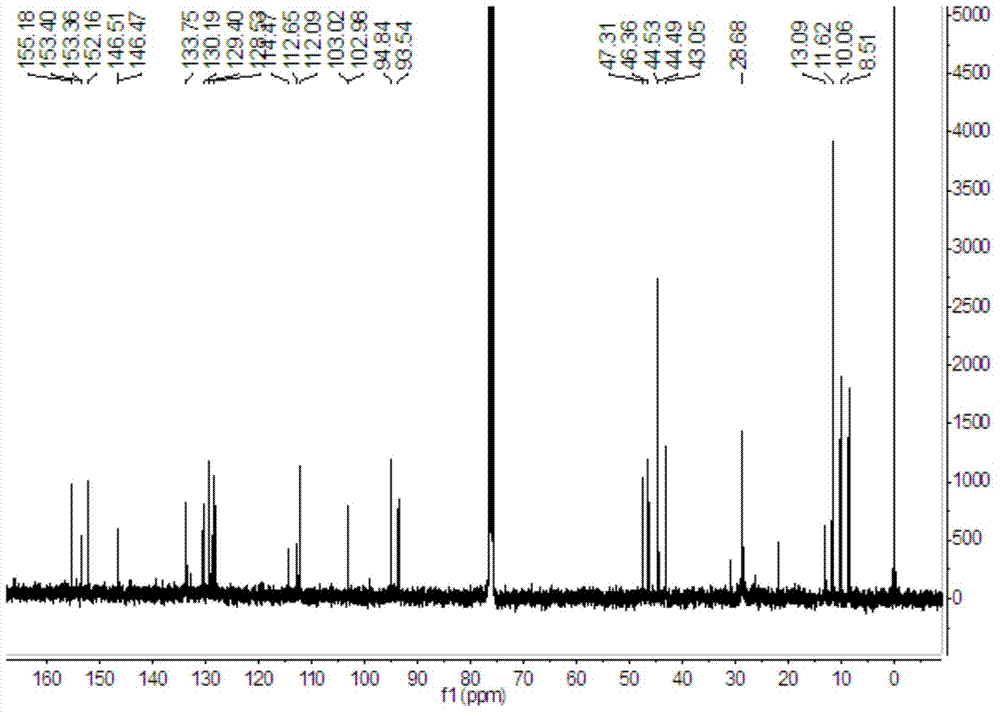
[0055] 1. Synthesis of intermediate 4-methoxy-1,2-o-phenylenediamine
[0056]
[0057] 4-Methoxy-2-nitroaniline (16.8g, 0.1mol) was dissolved in 200mL of methanol, 1.68g of palladium carbon and 22mL of hydrazine hydrate were added, and reacted at 60°C for 6 hours to obtain the red oily intermediate 4-methoxy- 1,2-o-phenylenediamine.
[0058] 2. Synthesis of intermediate 6-methoxyquinoxaline
[0059]
[0060] Dissolve 4-methoxy-1,2-o-phenylenediamine in 350mL of acetonitrile, add dropwise 32mL of 40% aqueous solution of glyoxal, react at 60°C for 6 hours, evaporate the solvent, and collect the distillate by vacuum distillation or column chromatography. Yellow needle intermediate 6-methoxyquinoxaline.
[0061] 3. Synthesis of intermediate 1,4-diethyl-6-methoxy-1,2,3,4-tetrahydroquinoxaline
[0062]
[0063] 6-Methoxyquinoxaline (5.5g, 0.034mol) was dissolved in 150mL of anhydrous toluene, sodium borohydride (13.2g, 0.35mol) was slowly added at 5°C, and then glacial a...
Embodiment 2
[0071] Embodiment 2: Spectral properties of rhodamine dyes in different solvents
[0072] 1. Absorption spectra of rhodamine dyes in different solvents
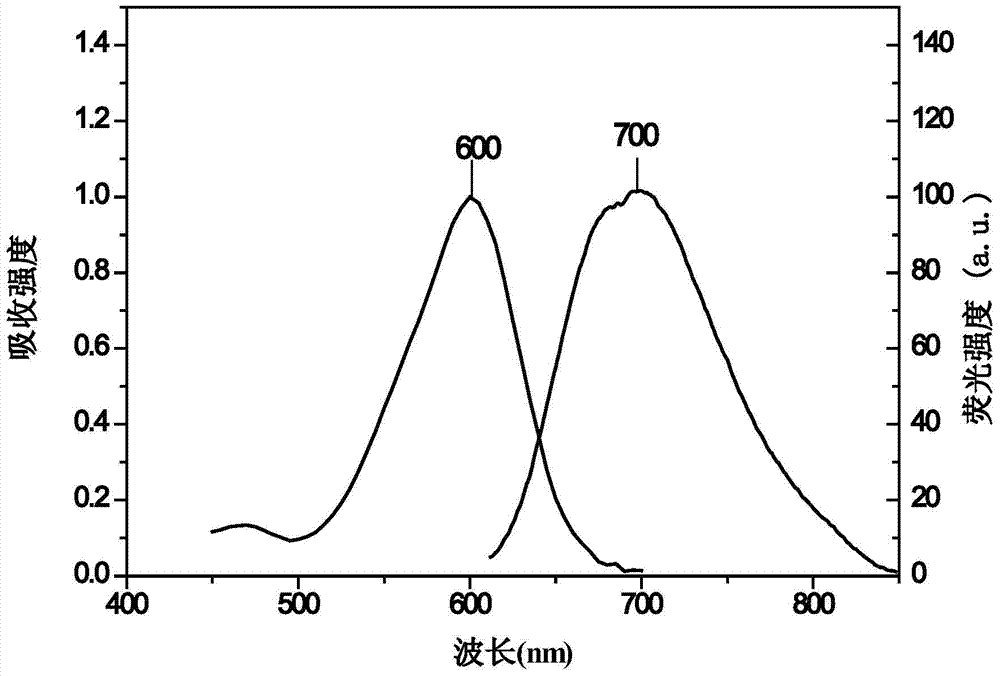
[0073] Take 17.1mg rhodamine dye and dissolve it in 7.07mL DMSO to make a concentration of 5mmol L -1 mother liquor. Pipette 8 μL of mother liquor into a sample bottle, and make 4 mL of solution with different solvents, the concentration is 10 μmol L -1 . Determination of the absorption spectra of rhodamine dyes in different solvents as attached Figure 4 shown. It can be seen from the figure that the rhodamine dye has a large absorbance in polar solvents, and the molar extinction coefficient (ε) of the dye in the aqueous solution is 34486mol -1 ·L·cm -1 .
[0074] 2. Fluorescence emission spectra of rhodamine dyes in different solvents
[0075] Determination of 10μmol·L in different solvents -1 The fluorescence emission spectrum of the rhodamine solution is attached Figure 5 shown. It can be seen from the figure ...
Embodiment 3
[0076] Embodiment 3: Spectral properties of rhodamine dyes in different pH aqueous solutions
[0077] 1. Absorption spectra of rhodamine dyes in different pH aqueous solutions
[0078] Pipette 8 μL to a concentration of 5mmol·L -1 The mother liquor into the sample bottle, made into aqueous solutions with different pH values, the concentration is 10μmol L -1 . Determination of the absorption spectra of rhodamine dyes in different pH solutions as attached Image 6 shown. It can be seen from the figure that the configuration of rhodamine dyes changes when pH=1-3, and the configuration is relatively stable in aqueous solutions with pH≥4.
[0079] 2. Fluorescence emission spectra of rhodamine dyes in different pH aqueous solutions
[0080] Under the irradiation of 578nm excitation light, measure 10 μmol L in different pH solutions -1 The fluorescence emission spectrum of rhodamine dye is attached Figure 7 shown. It can be seen from the figure that pH has little effect on f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com