Preparation method of catalyst used for chlorine preparation, catalyst, and method used for preparing chlorine
一种催化剂、氯气的技术,应用在催化剂活化/制备、非均相催化剂化学元素、化学仪器和方法等方向,能够解决催化剂活性组分挥发流失、易飞温、床层飞温等问题,达到良好流动性和活性、良好抗粘结性、小磨损指数的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0058] Preparation of slurry A:
[0059] Weigh 2kg boric acid, 5kg potassium dichromate, put into 100kg deionized water, then use 2mol L -1 hydrochloric acid solution to adjust the pH to 0.3, and fully stirred at room temperature for 3 hours to obtain slurry A-1, in which 2.25 g of insoluble matter was obtained.
[0060] Weigh 2kg boric acid, 10kg chromium trioxide, put into 100kg deionized water, and then use 0.1molL -1 The pH of the KOH solution was adjusted to 3.0, and it was fully stirred at room temperature for 3 hours to obtain slurry A-2, in which 4.93 g of insoluble matter was obtained.
[0061] Weigh 5kg of boric acid and 2kg of chromium chloride hexahydrate, put them into 100kg of deionized water, and then use 1molL -1 The pH of the nitric acid solution was adjusted to 1.0, and the mixture was fully stirred at room temperature for 3 hours to obtain slurry A-3, in which 1.71 g of insoluble matter was obtained.
[0062] Preparation of slurry B:
[0063] Put 35kg of...
Embodiment 1
[0067] Catalyst preparation:
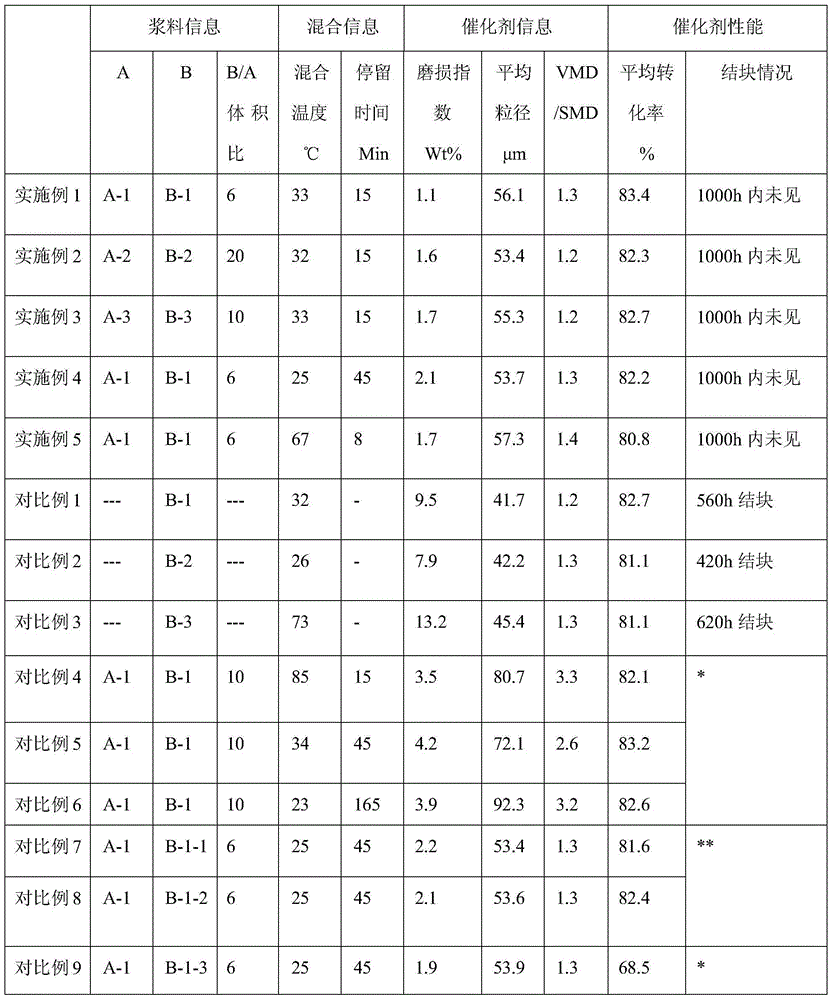
[0068] At room temperature (about 25°C), slowly and continuously add 3L of slurry A-1 into 18L of vigorously stirred slurry B-1, the mixing temperature is 33°C, the residence time is about 15min, and the mixed slurry is used The twin-screw pump is fed into the centrifugal spray drying tower at a rate of 15L / h, and the materials are collected by a cyclone separator and a bag filter after the spray drying tower. The materials collected by the cyclone separator were roasted in a muffle furnace. The temperature rise rate of the muffle furnace was 2°C / min, and the roasting temperature was 500°C. The roasting time was about 1 hour and then cooled to obtain 8.6kg of finished catalyst.
[0069] After analysis, the average particle size of the catalyst is 56.1 μm, VMD / SMD=1.3, and the wear index is 1.1%. Catalyst performance test:
[0070] Put 1 kg of catalyst into a fluidized bed reactor with an inner diameter of 30mm and a height of 700mm, preheat the...
Embodiment 2
[0072] Catalyst preparation:
[0073] At room temperature (about 25°C), slowly and continuously add 1L of slurry A-2 into 20L of vigorously stirred slurry B-2, the mixing temperature is 32°C, the residence time is about 15min, and then the process is carried out as in Example 1 Spray drying, cyclone separation, bag dust removal to collect materials under the same conditions, cooling after calcination to obtain 8.1 kg of finished catalyst.
[0074] After analysis, the average particle size of the catalyst was 53.4 μm, VMD / SMD=1.2, and the wear index was 1.6%. Catalyst performance test:
[0075] The catalyst performance test was carried out as in Example 1, and the detailed information is listed in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| wear volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com