Catalyst for catalyzing oxidation of cyclohexane and preparation method of catalyst
A technology of catalyst and cyclohexane, applied in the direction of physical/chemical process catalyst, organic compound/hydride/coordination complex catalyst, chemical instrument and method, etc., can solve the problem of high catalytic efficiency of porphyrin, and achieve convenient Recovery and recyclability, high catalytic efficiency, high efficiency effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
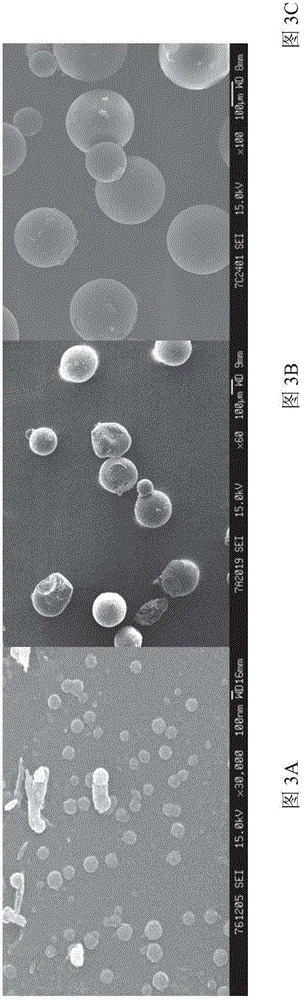
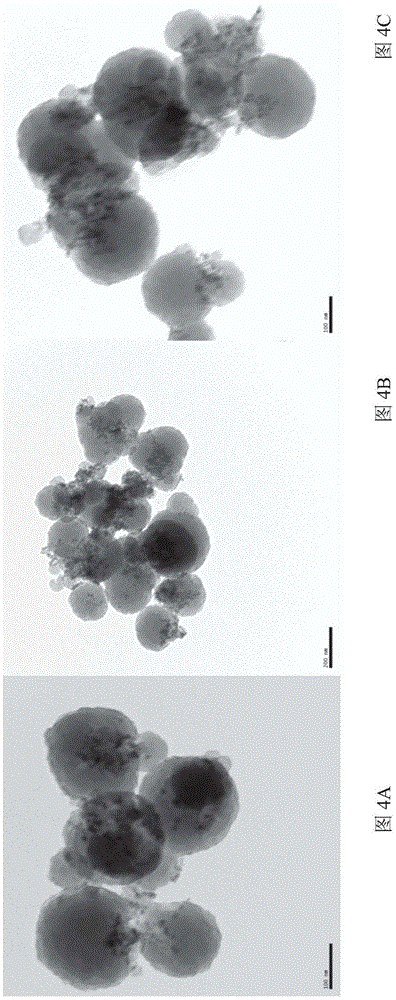
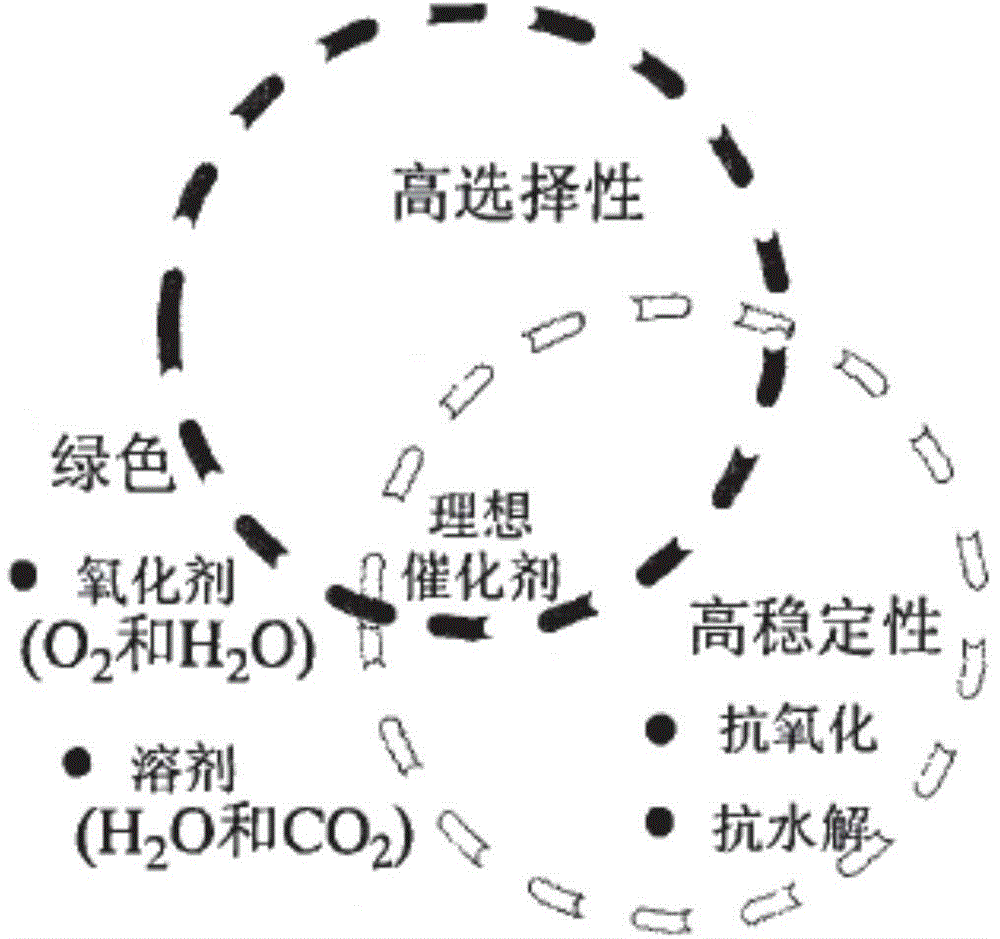
Image
Examples
example 1
[0108] (A) Preparation of 5(p-propionate)phenyl-10,15,20-tripyridylporphyrin (EPTPyP):
[0109] React p-hydroxybenzaldehyde, propionic acid, acetic anhydride, pyrrole and 4-pyridine formaldehyde with ethanol, where the mass of p-hydroxybenzaldehyde is used as a benchmark, and 1g of p-hydroxybenzaldehyde requires 100 to 120 mL of propionic acid and 5 to 50 mL of acetic anhydride. 8mL, pyrrole 2~4mL, 4-pyridinecarbaldehyde 2~4mL, ethanol 100~150mL, after standing for 12~18 hours, suction filtration, washing, and column chromatography to obtain 5(p-propionate)phenyl-10, 15,20-tripyridylporphyrin (EPTPyP).
[0110] (B) Preparation of 5(p-hydroxy)phenyl-10,15,20-tripyridylporphyrin (HPTPyP):
[0111] The 5 (p-propionate group) phenyl-10,15,20-tripyridyl porphyrin (EPTPyP) prepared in step (A) is mixed with the methanol solution of chloroform and NaOH and magnetically stirred, and reacted at room temperature After 12 hours, washing with water, centrifugation to remove water and dr...
example 2
[0119] (A) Preparation of 5(p-propionate)phenyl-10,15,20-tripyridylporphyrin (EPTPyP):
[0120] Weigh p-hydroxybenzaldehyde and place it in a reaction vessel, then add propionic acid and acetic anhydride, stir and heat with magnetic force, the reaction temperature is 120-130°C, add pyrrole and 4-pyridine formaldehyde dropwise, heat up to 145-145°C and reflux for 2.5 ~3.5 hours, then cooled to 75~85°C, added ethanol while stirring, left overnight, filtered, washed, and separated by column chromatography to obtain purple crystal 5(p-propionate)phenyl-10,15,20-tri Pyridyl porphyrin: Based on the quality of p-hydroxybenzaldehyde, the volume of propionic acid needed to react 1g of p-hydroxybenzaldehyde is 100-120mL, the volume of acetic anhydride is 5-8mL, and the volume of pyrrole is 2-4mL. The required volume of 4-pyridinecarbaldehyde is 2-4mL, and the required volume of ethanol is 100-150mL; the eluent used for column chromatography separation is a mixture of chloroform and etha...
example 3
[0130] (A) Synthesis of 5-(p-hydroxy)phenyl-10,15,20-tripyridyl porphyrin (MnHPTPyP) coordinated by metal manganese:
[0131] Add 1.386g p-Hydroxybenzaldehyde, 180ml propionic acid, 7ml acetic anhydride in 500ml three-necked bottle, magnetic stirring and heating. The temperature of the oil bath is controlled at about 125°C. 3ml freshly distilled pyrrole and 3ml 4-pyridinecarbaldehyde diluted with 10ml propionic acid were added dropwise with two dropping funnels respectively. After 20 minutes of addition, the solution gradually turns brownish black. Keep stirring, raise the temperature to 140°C and reflux for 3 hours, then evaporate most of the propionic acid and cool to 80°C. 50ml of ethanol was added under stirring and left overnight. Suction filter and wash the crystals with ethanol to obtain dark purple crystals.
[0132] Using a mixed solvent of chloroform and ethanol with a volume ratio of 60:1 as the eluent, the third color band was collected by separation on a silica...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com