Cosmetic additives and cosmetics
A technology of cosmetic additives and cosmetics, applied in the direction of cosmetic preparations, cosmetics, dressing preparations, etc., can solve the problems of lack of anti-inflammatory effects, etc., and achieve the effect of eliminating hydroxyl free radical activity, preventing damage, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
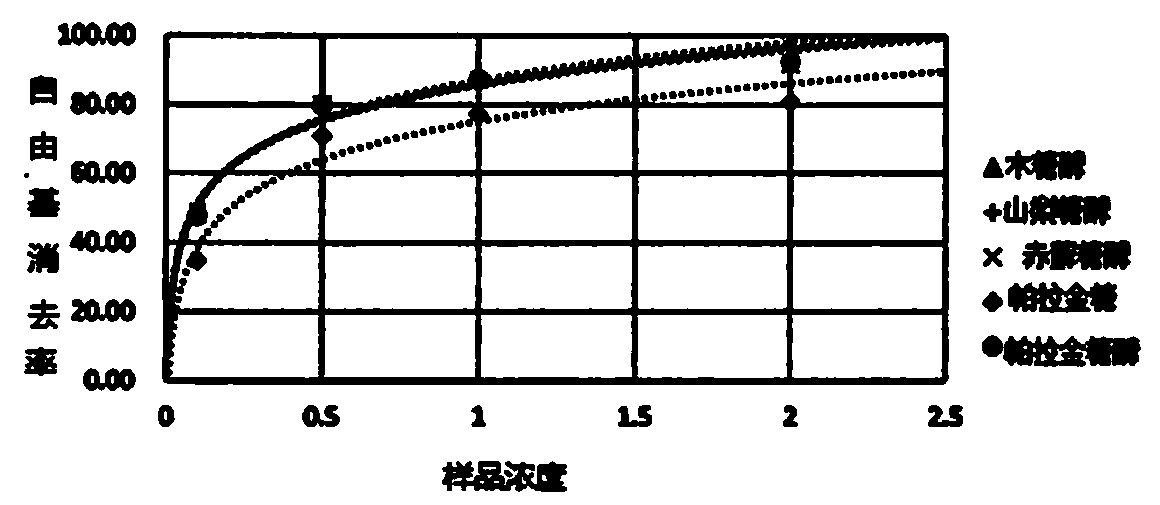
[0036] Experimental Example 1: Evaluation of Hydroxyl Radical Scavenging Activity
[0037]According to the deoxyribose method (Halliwell, 1981), the elimination rate of hydroxyl radicals of isomalt, 6-fructose-α-glucoside, erythritol, xylitol and sorbitol was obtained.
[0038] Specifically, first in purified water, add 33.6mM-deoxyribose (final concentration 4.2mM), 200μL-iron(III) oxide / 1.04mM-EDTA solution (final concentration of Fe is 100μM), 120mM-phosphate buffer (pH7. 4, final concentration 15mM), 1mM-ascorbic acid (final concentration 150μM), 0.02% hydrogen peroxide, 50mM-NaOH, 1%TBA (thiobarbituric acid) / 50mM-NaOH solution, and 2.8%TCA (trichloro Acetic acid), prepared as a reagent. In this reagent, various sugar alcohols (isomalt, isomalt, erythritol, xylitol, sorbitol) are adjusted according to the concentrations shown in Table 1 and Table 2. At the same time, purified water is used in this reagent instead of the sugar alcohol solution as the base liquid.
[0039...
experiment example 2
[0046] Experimental example 2: Verification of the effect of inhibiting the generation of active oxygen on hair
[0047] After the hair is irradiated by ultraviolet rays, it will generate reactive oxygen species such as hydrogen peroxide and hydroxyl radicals, which will cause an oxidation chain reaction to promote the oxidation of cystine to sulfalanine, thereby promoting the formation of disulfide bonds in hair. resulting in damage to the epidermis of the hair. This experiment used the reactive oxygen fluorescence reaction of fluorescein to verify the effect of isomalt and isomalt on the prevention of hair reactive oxygen species. Hydroxyphenyl fluorescein (HPF for short) has no fluorescence in neutral aqueous solution, but after reacting with oxides with the same high reactivity as active oxygen, it generates a strong fluorescent compound fluorescein (excitation wavelength 490nm, fluorescence wavelength 515nm), And an increase in fluorescence intensity can be observed. HP...
experiment example 3
[0051] Experimental Example 3: Verification of the protective effect against hair damage under UV irradiation
[0052] Take 1ml of the aqueous solution mixed with water and 1% isomalt into a 35mm glass shallow dish, and then immerse 10 hairs of healthy women between the ages of 20 and 30 with a length of 1cm, respectively, into the water and 1% isomalt aqueous solution. Separate hair from root to root so they don't overlap. Next, after 20 minutes of UV (wavelength 280-400nm) irradiation from above the glass tray, the state of the cuticle layer of the hair was observed with a microscope and electron micrographs were taken. At the same time, as a comparison reference, the hair without UV irradiation was also observed under a microscope for its epidermis state and microscopic photos were taken. Figure 5 It is a photomicrograph of hair without UV irradiation, Figure 6 It is an electron micrograph after soaking in 1% isomalt aqueous solution and carrying out UV irradiation, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com