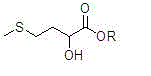
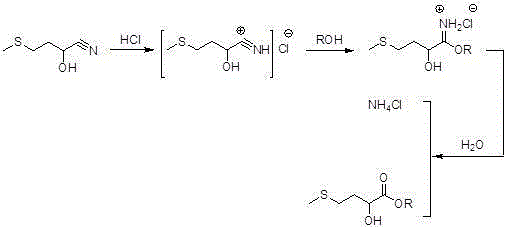
Preparation method for high-purity D,L-2-hydroxyl-4-methylthiobutyrate
A technology of methylthiobutyrate and methylthiobutyronitrile is applied in the field of preparation of D,L-2-hydroxy-4-methylthiobutyrate, which can solve the problem of 4-methylthiobutyrate Low yield, preparation method needs to be improved, product purity is not high, etc., to avoid the formation of dimers and polymers, easy to separate, simple process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] In a four-necked round-bottomed flask equipped with mechanical stirring, a thermometer and its exhaust gas absorption, add 530.1 grams of D,L-2-hydroxyl-4-methylthiobutyronitrile with a purity of 99% and 2120 grams of methanol hydrochloride (which contains hydrochloric acid 30 wt.%), stirred and heated to 65°C for 5 hours; high performance liquid chromatography analysis D, L-2-hydroxy-4-methylthiobutyronitrile residue was less than 100ppm, distilled unreacted methanol hydrochloride, Then add 86 grams of deionized water, stir, and precipitate a large amount of inorganic ammonium salt; then distill water under reduced pressure to almost anhydrous, cool, stand still, and centrifuge to remove salt, containing D,L-2-hydroxy-4-methylthio The ammonium chloride of methyl butyrate is washed with recovered methanol hydrochloride and dried to obtain ammonium chloride with a purity of 99%, and the washing liquid is recycled to the next D,L-2-hydroxy-4-methylthiobutyronitrile ester ...
Embodiment 2
[0030] In a four-necked round-bottomed flask equipped with mechanical stirring, a thermometer and its tail gas absorption, add 530.1 grams of D,L-2-hydroxyl-4-methylthiobutyronitrile with a purity of 99% and 2650 grams of hydrochloric acid ethanol (which contains hydrochloric acid 25 wt.%), stirred and heated to 65°C for 6 hours; high performance liquid chromatography analysis D, L-2-hydroxy-4-methylthiobutyronitrile residue was less than 50ppm, distilled unreacted ethanol hydrochloride, Then add 86 grams of deionized water, stir, and precipitate a large amount of inorganic ammonium salt; then distill water under reduced pressure to almost anhydrous, cool, stand still, and centrifuge to remove salt, containing D,L-2-hydroxy-4-methylthio The ammonium chloride of ethyl butyrate is washed with recovered hydrochloric acid ethanol, dried to obtain ammonium chloride with a purity of 99%, and the washing liquid is recycled to the next D, L-2-hydroxy-4-methylthiobutyronitrile ester Ch...
Embodiment 3
[0033] In a four-necked round-bottomed flask equipped with mechanical stirring, a thermometer and its exhaust gas absorption, add 530.1 grams of D with a purity of 99%, L-2-hydroxyl-4-methylthiobutyronitrile and 2650 grams of isopropanol hydrochloride (wherein Containing hydrochloric acid 25 wt.%), stirred and heated to 75 ° C for 5 hours, the residual amount of D, L-2-hydroxy-4-methylthiobutyronitrile was analyzed by high performance liquid chromatography was less than 50ppm, unreacted hydrochloric acid was evaporated Isopropanol, then add 86 grams of deionized water, stir, and precipitate a large amount of inorganic ammonium salt; then distill water under reduced pressure to almost anhydrous, cool, stand still, and centrifuge to remove salt, containing D,L-2-hydroxy-4 - The ammonium chloride of isopropyl methylthiobutyrate is washed with recovered isopropanol hydrochloride and dried to obtain ammonium chloride with a purity of 99%, and the washing liquid is circulated to the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com