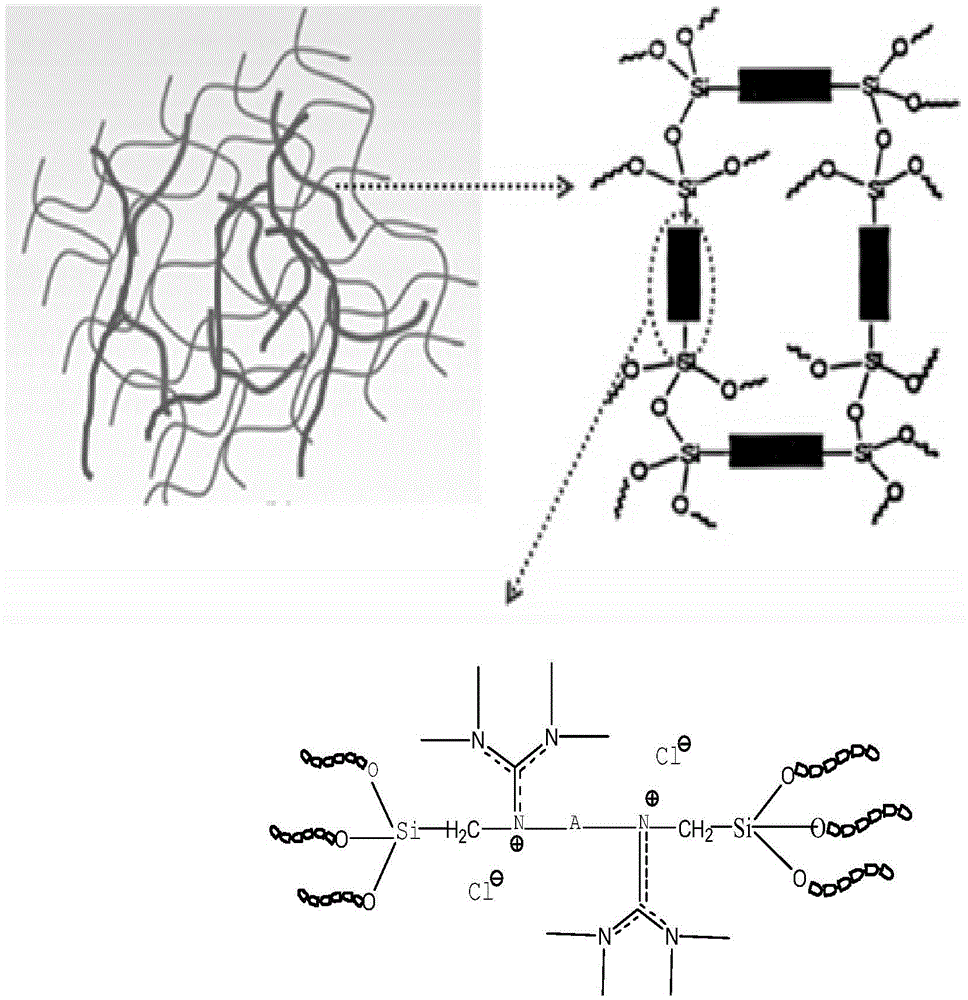
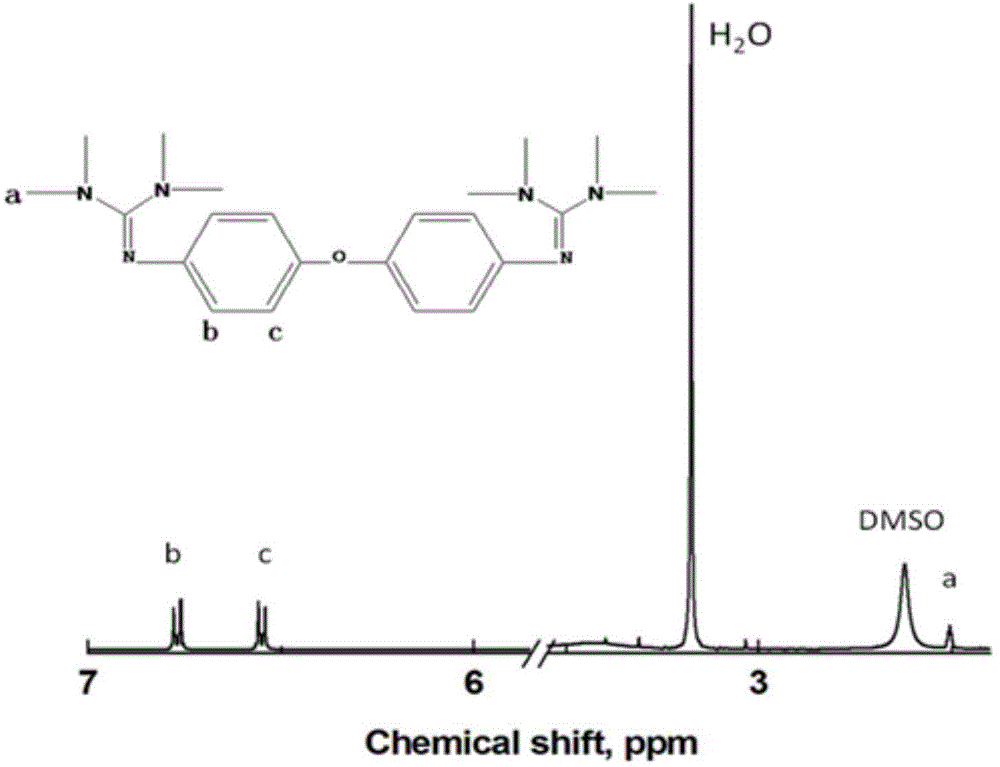
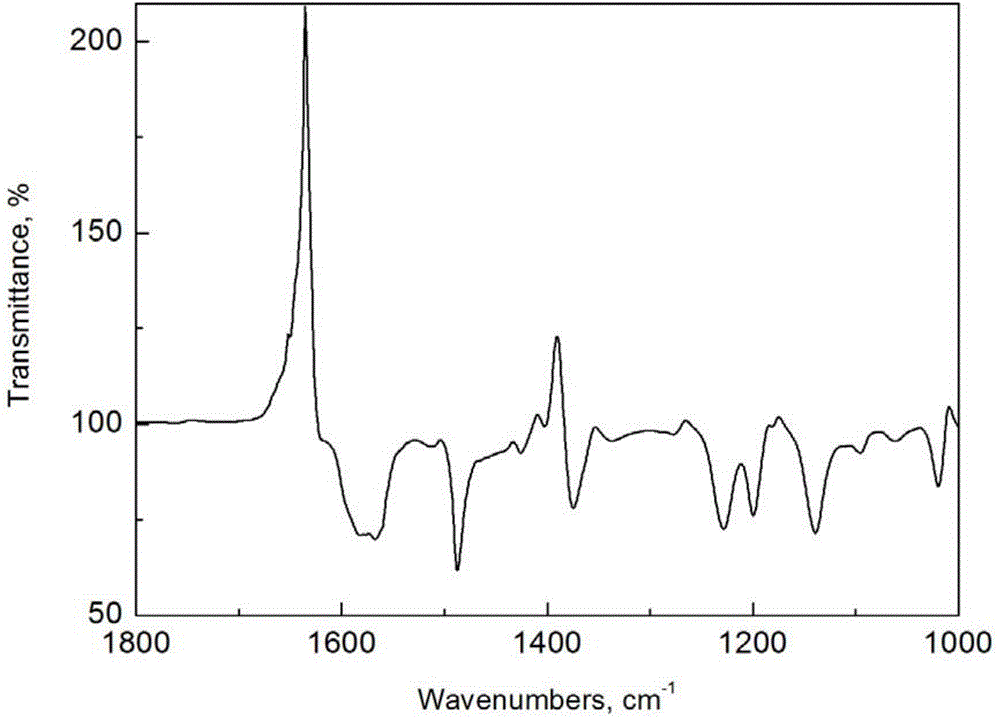
Crosslinking type polymer anionic membrane and preparation method thereof
An anion membrane, polymer technology, applied in electrical components, circuits, fuel cell parts and other directions, can solve the problems of expensive, high conductivity, low water absorption, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 (Solvent is CH 2 Cl 2 , 55℃ ionization reaction for 4 hours, DC=0.84)
[0042] Preparation of guanidine salt: under nitrogen atmosphere, dissolve tetramethylurea in dichloromethane, add oxalyl chloride dropwise, slowly increase the temperature to 60℃ and react for 1.5 hours, remove the solvent to obtain white solid Vilsmeyer salt, (tetramethylurea : Dichloromethane: oxalyl chloride = 9.6ml: 10ml: 10.2ml);
[0043] Dissolve 1 mole fraction of guanidine salt in 5 mole fraction of acetonitrile, add 0.4 mole fraction of diaminodiphenyl ether, slowly raise the temperature to 80°C and react for 24 hours. After the reaction is completed, add the reaction mixture to the 5M NaOH solution vigorously After stirring for 6 hours, the organic phase was separated, and the organic phase and solvent were removed by distillation under reduced pressure to obtain the yellow-brown viscous ionizing reagent diguanidyl diphenyl ether.
[0044] Ionization of the polymer membrane backbone: T...
Embodiment 2
[0049] Example 2 (solvent is toluene, ionization reaction at room temperature for 12 hours, DC=0.84)
[0050] Preparation of guanidine salt: Under nitrogen atmosphere, dissolve tetramethylurea in toluene, add oxalyl chloride dropwise, slowly increase the temperature to 60°C and react for 1.5 hours, distill off the solvent to obtain a white solid, which is Vilsmeyer salt (tetramethylurea: Toluene: oxalyl chloride = 9.6ml: 20ml: 10.2ml);
[0051] Dissolve 1 mole fraction of guanidine salt in 5 mole fraction of acetonitrile, add 0.4 mole fraction of diaminodiphenyl ether, slowly raise the temperature to 80°C and react for 24 hours. After the reaction is completed, add the reaction mixture to the 5M NaOH solution vigorously After stirring for 6 hours, the organic phase was separated, and the organic phase and solvent were removed by distillation under reduced pressure to obtain the yellow-brown viscous ionizing reagent diguanidyl diphenyl ether.
[0052] Ionization of polymer membrane b...
Embodiment 3
[0057] Example 3 (Solvent is acetonitrile, ionized reaction at 55°C for 4 hours, DC=0.99)
[0058] Preparation of guanidine salt: under nitrogen atmosphere, dissolve tetramethylurea in acetonitrile, add oxalyl chloride dropwise, slowly increase the temperature to 60°C and react for 1.5 hours, distill off the solvent to obtain a white solid, which is Vilsmeyer salt (tetramethylurea: Acetonitrile: oxalyl chloride = 9.6ml: 10ml: 10.2ml);
[0059] Dissolve 1 mole fraction of guanidine salt in 5 mole fraction of acetonitrile, add 0.4 mole fraction of diaminodiphenyl ether, slowly raise the temperature to 80°C and react for 24 hours. After the reaction is completed, add the reaction mixture to the 5M NaOH solution vigorously After stirring for 6 hours, the organic phase was separated, and the organic phase and solvent were removed by distillation under reduced pressure to obtain the yellow-brown viscous ionizing reagent diguanidyl diphenyl ether.
[0060] Ionization of the polymer membran...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com