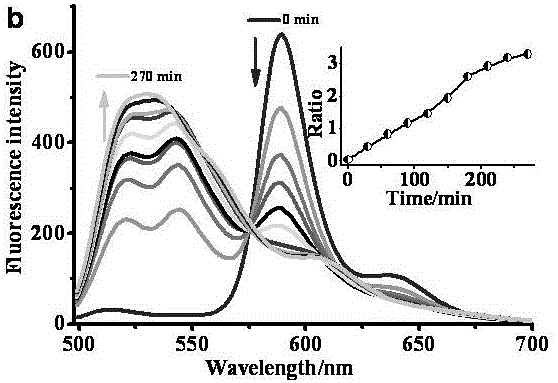
Fluorescent probe for monitoring lipid peroxidation processes in different subcellular organelles
A lipid peroxidation and fluorescent probe technology, applied in the field of fluorescent probes, can solve the problems of high price, cumbersome synthesis and preparation process, and high cost, and achieve the effects of easy long-term storage, sensitive response, and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
[0037] Add 500 mg of tetramethylfluoroborate and 129 mL of cinnamaldehyde into two-necked reaction flasks, then add 500 mL of piperidine as a catalyst, and 4? After removing toluene, silica gel column separation (eluent: dichloromethane: n-hexane = 1:4) gave 155 mg of a purple solid with a yield of 23%.
Embodiment 2
[0039]
[0040] Add 500 mg of tetramethylfluoroborate and 94 mL of cinnamaldehyde into two-necked reaction flasks, then add 500 mL of piperidine as a catalyst, and 4? After removing toluene, silica gel column separation (eluent: dichloromethane: n-hexane = 1:4) yielded 194 mg of purple solid with a yield of 31%.
[0041]
[0042] Dissolve 100 mg of the product obtained in the previous step in DMSO, add excess morpholine 100 mL, 24 mg K 2 CO 3 and 30mg KI, reacted at 60°C for 12h, washed the reactant with CH 2 Cl 2Extract, keep the organic phase, dry over anhydrous magnesium sulfate, spin dry under reduced pressure, and separate on a silica gel column (dichloromethane: methanol = 20:1 as the eluent) to obtain 81 mg of a purple solid with a yield of 75%.
Embodiment 3
[0044]
[0045] Add 500 mg of tetramethylfluoroborate and 88 mL of cinnamaldehyde into two-necked reaction flasks, then add 500 mL of piperidine as a catalyst, and 4? After toluene was removed, silica gel column separation (eluent: dichloromethane: n-hexane = 1:4) gave 217 mg of a purple solid with a yield of 35%.
[0046]
[0047] Dissolve 100 mg of the product obtained in the previous step in acetone, add 50 mg of excess tertiary amine, KI 30 mg, react at 60°C for 12 h, concentrate the reaction solution, and add diethyl ether, a dark purple solid precipitates out, which is the target product. Dissolve the fluorescent probe in methanol, add 1000 mg of potassium periodate, stir at 50°C for 24 hours, then spin dry the reaction solution under reduced pressure, dissolve it in a small amount of dichloromethane, filter to remove inorganic salts, keep the filtrate, and spin dry again under reduced pressure , to obtain 141 mg of dark purple solid, which is a fluorescent probe w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com