Dibenzosuberan enone derivatives and preparation method therefor and application thereof
A technology of dibenzocycloheptenone and its derivatives, which is applied in the field of novel dibenzocycloheptenone derivatives and their preparation and application, can solve problems such as cis-trans configurational isomerization, achieve optimal performance, High thermal stability, good film-forming effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
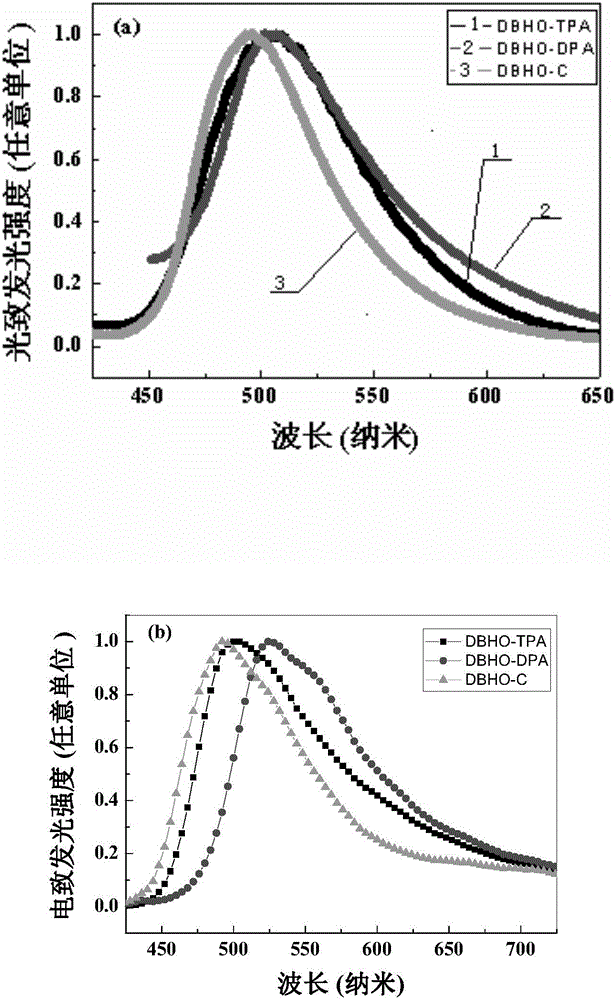
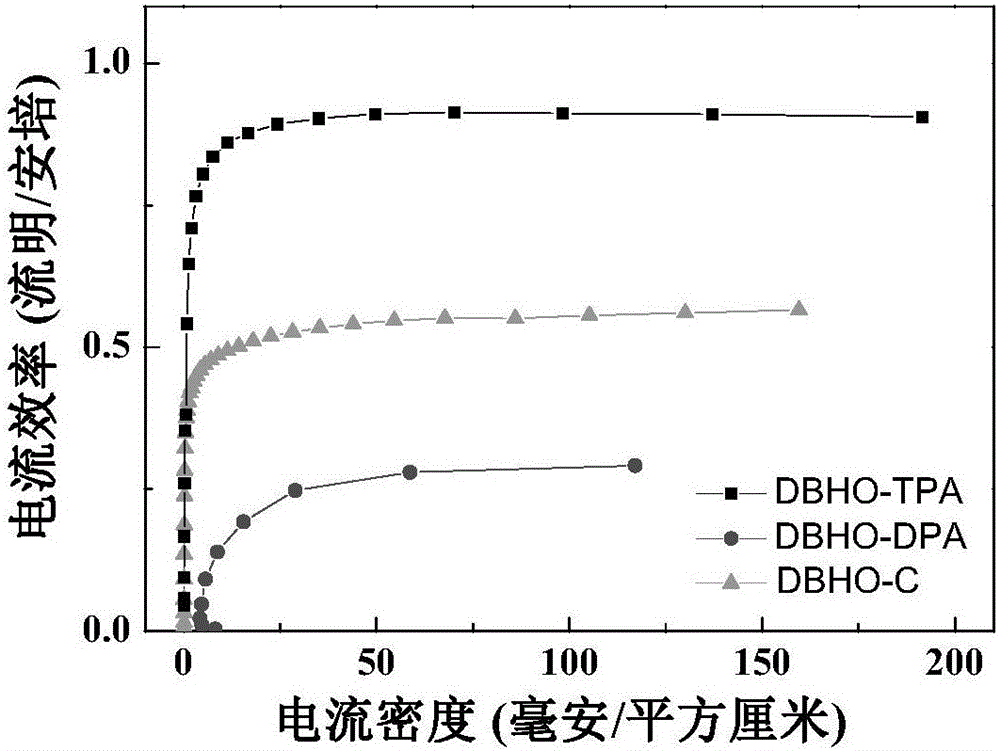
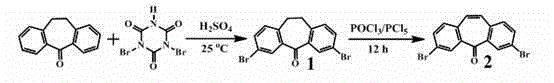
[0038] Synthesis of 3,7-bis-(4-dianilinobenzene)-dibenzocyclohepten-5-one (DBHO-TPA): 3,7-Dibromodibenzo[a,d]cycloheptenene -5-ketone (2) (49.14mg, 0.135mmol) and 4-boronate triphenylamine (3) (100mg, 0.27mmol) were dissolved in tetrahydrofuran, and then 2M K was added 2 CO 3 Adjust the pH to 8. Then the whole system is evacuated and filled with nitrogen, and under nitrogen condition, Pd(PPh 3 ) 4 (7.8 mg), and the mixed system was stirred under reflux at 70°C for 48 hours. After the reaction was finished, the solvent was removed under reduced pressure, dichloromethane was added to dissolve, and the inorganic salt was removed by extraction with NaCl salt solution. The organic phase was dried over anhydrous magnesium sulfate, filtered, and the filtrate was removed by rotary evaporation. The crude product was subjected to silica gel column chromatography ( Petroleum ether-ethyl acetate) to obtain 57 mg of a yellow solid product (DBHO-TPA), with a yield of 61%. The syntheti...
Embodiment 2
[0042] Synthesis of 3,7-di-diphenylamino-dibenzocyclohepten-5-one (DBHO-DPA): Compound 3,7-dibromodibenzo[a,d]cyclohepten- 5-ketone (2) (245mg, 0.68mmol) and diphenylamine (230mg, 1.36mmol) were dissolved in toluene, and sodium tert-butyloxide (146.4mg, 1.52mmol) was added thereto, filled with nitrogen, and stirred at room temperature for half After hours, add Pd 2 (dba) 3 (19.55mg, 0.03mmol) and P( t -Bu) 3 (0.03 mmol), heated to reflux at 100° C. for 16 hours under a nitrogen atmosphere. After the reaction, the solvent was removed under reduced pressure, ethyl acetate was added to dissolve, NaCl salt solution was extracted, and the organic phase was dried with anhydrous magnesium sulfate. Through silica gel column chromatography (petroleum ether-ethyl acetate), 122 mg of a yellow solid product (DBHO-DPA) was obtained, with a yield of 33%. The synthetic route of DBHO-DPA is shown in Table 1.
[0043] The structural formula is as follows:
[0044] .
Embodiment 3
[0046] Synthesis of 3,7-di-carbazole-9-benzocyclohepten-5-one (DBHO-C): Compound 3,7-dibromodibenzo[a,d]cyclohepten-5- Ketone (2) (245mg, 0.68mmol) and carbazole (227mg, 1.36mmol) were dissolved in toluene, then sodium tert-butyloxide (146.4mg, 1.52mmol) was added, filled with nitrogen, and stirred at room temperature for half an hour , join Pd 2 (dba) 3 (19.55mg, 0.03mmol) and P( t -Bu) 3 (0.03mmol), heated to reflux at 100°C for 16 hours under a nitrogen atmosphere. After the reaction, the solvent was removed under reduced pressure, ethyl acetate was added to dissolve, NaCl salt solution was extracted, and the organic phase was dried with anhydrous magnesium sulfate. Through silica gel column chromatography (petroleum ether-ethyl acetate), 200 mg of a yellow solid product (DBHO-C) was obtained, with a yield of 87.5%. The synthetic route of DBHO-C is shown in Table 1
[0047] The structural formula is as follows:
[0048] .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com