Lipoic acid liquid preparation and preparation method thereof
A technology of liquid preparations and lipoic acid, which is applied in the field of medicine, can solve the problems of increasing the risk of adverse reactions and clinical medication, low content of lipoic acid injection, and unsatisfactory stability effects, etc., to achieve faster dissolution and environmental tolerance Good resistance, prevent degradation and deterioration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
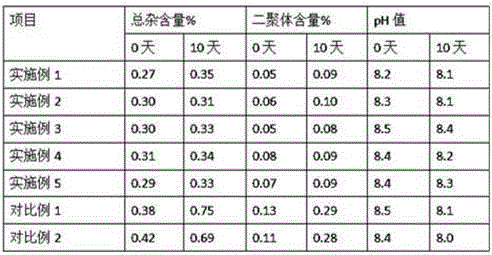
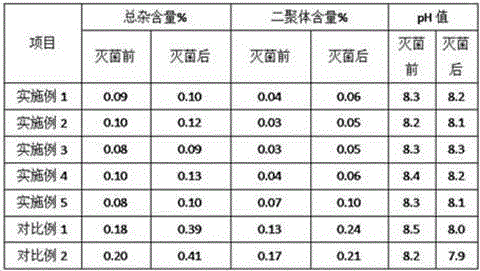
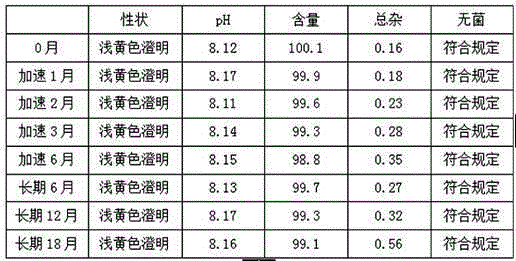
Embodiment 1
[0033]Example 1 Each 0.3g / 10mL, 1000
[0034] Lipoic Acid 300g
[0035] Lysine 350g
[0036] Disodium hydrogen phosphate 25g
[0037] Phosphoric acid to adjust pH8.0~8.5
[0038] Make up to 10L the remaining amount of water for injection.
[0039] Preparation:
[0040] 1) Mix lipoic acid and lysine in a blender evenly, set aside;
[0041] 2) Fill the liquid mixing tank with nitrogen saturation for 10-20 minutes, then add 70% of the prescription water for injection, add disodium hydrogen phosphate and stir to dissolve, control the temperature at 35-40°C, and then inject nitrogen saturation, Control the residual oxygen to be less than 2%;
[0042] 3) Add the mixture of lipoic acid and lysine in step 1 under stirring, after it is completely dissolved, add 0.1mol / L phosphoric acid dropwise to adjust the pH value to 8.0-8.5, add medicinal activated carbon and stir for 15 minutes, decarburize, Make up the water for injection to the full amount;
[0043] 4) After fine filtrat...
Embodiment 2
[0044] Example 2 Each 0.3g / 10mL, 1000
[0045] Lipoic Acid 300g
[0046] Lysine 400g
[0047] Disodium hydrogen phosphate 25g
[0048] Phosphoric acid to adjust pH8.0~8.5
[0049] D-Sorbitol 30g
[0050] Make up to 10L the remaining amount of water for injection.
[0051] Preparation:
[0052] 1) Mix lipoic acid and lysine in a blender evenly, set aside;
[0053] 2) Fill the liquid mixing tank with nitrogen saturation for 10-20 minutes, then add 70% of the prescription amount of water for injection, add disodium hydrogen phosphate and D-sorbitol and stir to dissolve, control the temperature at 35-40°C, and then add Saturated with nitrogen, control the residual oxygen to less than 2%;
[0054] 3) Add the mixture of lipoic acid and lysine in step 1 under stirring, after it is completely dissolved, add 0.1mol / L phosphoric acid dropwise to adjust the pH value to 8.1-8.3, add medicinal activated carbon and stir for 15 minutes, decarburize, Make up the water for injection ...
Embodiment 3
[0056] Example 3 Each 0.3g / 10mL, 1000
[0057] D-lipoic acid 300g
[0058] Lysine 360g
[0059] Disodium hydrogen phosphate 24g
[0060] Phosphoric acid to adjust pH8.1~8.3
[0061] D-Sorbitol 30g
[0062] Make up to 10L the remaining amount of water for injection.
[0063] Preparation:
[0064] 1) Mix D-lipoic acid and lysine in a blender evenly, set aside;
[0065] 2) Fill the liquid mixing tank with nitrogen saturation for 10-20 minutes, then add 70% of the prescription amount of water for injection, add disodium hydrogen phosphate and D-sorbitol and stir to dissolve, control the temperature at 35-40°C, and then add Saturated with nitrogen, control the residual oxygen to less than 2%;
[0066] 3) Add the mixture of D-lipoic acid and lysine in step 1 under stirring, after it is completely dissolved, add 0.1mol / L phosphoric acid dropwise to adjust the pH value to 8.1-8.3, add medicinal activated carbon and stir for 15 minutes, remove Carbon, make up water for inject...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com