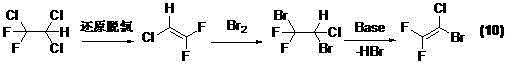Preparation method of 1-bromo-1-chloro-2,2-difluoroethylene
A technology of difluoroethylene and difluoroethane, which is applied in the field of preparation of fluorine-containing organic intermediates, can solve problems such as waste of resources, and achieve the effects of fast reaction speed, environmental friendliness, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] A preparation method of 1-bromo-1-chloro-2,2-difluoroethylene, which comprises the following steps:
[0061] (1) In an inert solvent, difluorotrichloroethane is used as raw material to carry out reductive dechlorination reaction with zinc powder to obtain 2-chloro-1,1-difluoroethylene;
[0062] The inert solvent of the present invention is one or a combination of alcohol solvents or amide solvents or ether solvents or acid anhydride solvents or oxygen-nitrogen-containing compound solvents or sulfur-containing compound solvents; The volume ratio of ethyl chloride is 1-10:1;
[0063] The volume ratio of the inert solvent in the present invention to difluorotrichloroethane is preferably 2-5:1;
[0064] In step (1) of the present invention, the alcohol solvent includes one or a combination of methanol, ethanol, propanol, butanol, ethylene glycol, glycerin; the amide solvent includes N,N-dimethylformaldehyde One or a combination of amides, N,N-dimethylacetamide; the ether ...
Embodiment 1
[0102] Zinc powder pretreatment:
[0103] Stir 2.4㎏ zinc powder with 6L 2% hydrochloric acid for 2 hours, filter to remove the acid, put the zinc powder in a 10L plastic beaker, wash once with 6L 2% hydrochloric acid, then wash three times with distilled water (5L each time), and then Wash twice with 95% ethanol (3L each time), and finally wash once with 3L anhydrous ether. Filter after each wash to remove washes. The treated zinc powder is thoroughly dried under the protection of nitrogen, and the lumps are ground in a mortar. The treated zinc powder is used under the protection of nitrogen.
[0104] Preparation of R12212:
[0105] A two-stage reflux condenser is connected to a 5-liter there-necked bottle equipped with a mechanical stirrer and a constant-pressure dropping funnel, wherein the first-stage reflux condenser is cooled by tap water, and the second-stage reflux condenser is condensed by circulating alcohol in a refrigerator. Control the temperature of the alcoho...
Embodiment 2
[0117] Preparation of R12212:
[0118] Install a 5-liter three-necked flask with a reflux condenser, a mechanical stirring device, and a constant-pressure dropping funnel, and add 2 liters of 1,4-dioxane (China Wulian Chemical Factory), activated zinc 325 grams of powder (analytically pure, Shanghai Shenxiang Chemical Reagent Co., Ltd.) and 11 grams of elemental iodine were reacted at 100 ° C, and 580 g of difluorotrichloroethane (R122, GC purity greater than 95%, Sinochem Lantian Group Co., Ltd. produces by-products of R123), and continued to react for 4 hours, and the generated R1122 was introduced into the second reaction bottle, and 60ml of solvent acetic acid and 493 grams of bromine were added to the second reaction bottle, and the reaction temperature was 40±5°C , and stir the reaction until the red color (bromine) of the reaction solution disappears, first wash three times with 300ml of water, and then the organic phase is distilled under reduced pressure with a water ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com