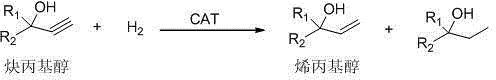Method for preparing allyl alcohol through selective hydrogenation of propargyl alcohol
A technology of propargyl alcohol and allyl alcohol, applied in hydrogenation preparation, organic chemistry, etc., can solve the problems of low selectivity, serious catalyst loss, and low selectivity of allyl alcohol, and achieve low catalyst unit consumption, The effect of low stirring speed and stable catalyst
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1 A kind of method for the selective hydrogenation of propargyl alcohol to prepare allyl alcohol
[0039] The propargyl alcohol is methyl butynol, and the allyl alcohol is methyl butenol.
[0040] Described method comprises the following steps:
[0041] (1) Feeding
[0042] A complex catalyst of 3g ammonium chloroplatinite and sodium triphenylphosphine intersulfonate, and 100g water were added to a 1L autoclave with magnetic stirring and a temperature controller, and then 200g methyl butynol was added; complexation The mass ratio of platinum chloride and sodium triphenylphosphine intersulfonate in the product is 0.01:1.
[0043] (2) Nitrogen, hydrogen replacement
[0044] It was replaced three times with nitrogen and three times with hydrogen.
[0045] (3) Hydrogenation reaction
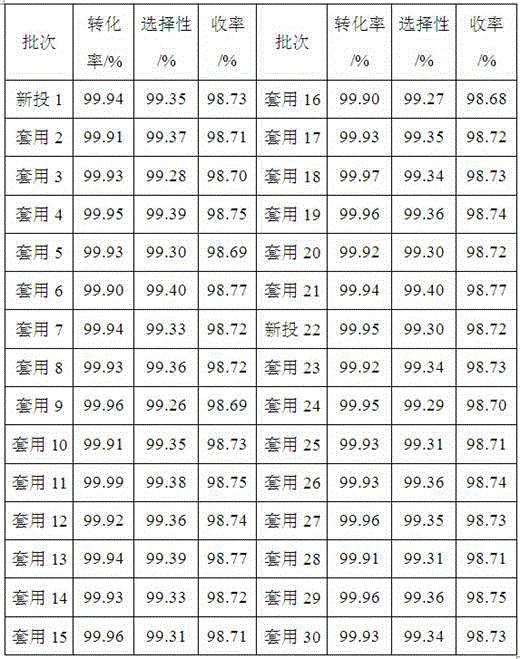
[0046] Heat to 70°C, increase the hydrogen pressure to 2.0MPa, stir at 200rpm, react for 8 hours, take a sample and analyze the conversion rate of methyl butynol >99.9%, stop...
Embodiment 2
[0051] Embodiment 2 A method for preparing allyl alcohol by selective hydrogenation of propargyl alcohol
[0052] The propargyl alcohol is dehydrolinalool, and the allyl alcohol is linalool.
[0053] Described method comprises the following steps:
[0054] (1) Feeding
[0055] Add 6g of palladium chloride and tri-(2,4-dimethyl-5-sodium sulfonate phenyl) phosphine complex catalyst, 200g of water into a 1L autoclave with magnetic stirring and temperature controller, and then Add 200 g of dehydrolinalool; the mass ratio of the complex palladium chloride to tris-(2,4-dimethyl-5-sulfonate sodium phenyl)phosphine is 0.008:1.
[0056] (2) Nitrogen, hydrogen replacement
[0057] It was replaced three times with nitrogen and three times with hydrogen.
[0058] (3) Hydrogenation reaction
[0059] Heating to 80°C, increasing the hydrogen pressure to 2.0MPa, stirring at 400rpm, and reacting for 15 hours, sampling and analyzing the conversion rate of dehydrolinalool>99.9%, and the r...
Embodiment 3
[0064] Embodiment 3 A kind of method for the selective hydrogenation of propargyl alcohol to prepare allyl alcohol
[0065] The propargyl alcohol is dehydronerolidol, and the allyl alcohol is nerolidol.
[0066] Described method comprises the following steps:
[0067] (1) Feeding
[0068] Add the complex catalyst of 8g palladium chloride and tris-(2-sodium sulfonate dibenzofuryl) phosphine, 200g water into a 1L autoclave with magnetic stirring and temperature controller, and then add dehydroneroli 200g of tertiary alcohol; the mass ratio of complex palladium chloride to tris-(2-sodium sulfonate dibenzofuryl)phosphine is 0.006:1.
[0069] (2) Nitrogen, hydrogen replacement
[0070] It was replaced three times with nitrogen and three times with hydrogen.
[0071] (3) Hydrogenation reaction
[0072] Heating to 80°C, increasing the hydrogen pressure to 2.0MPa, stirring at 400rpm, and reacting for 17 hours, sampling and analyzing the conversion rate of nerolidol >99.9%.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com