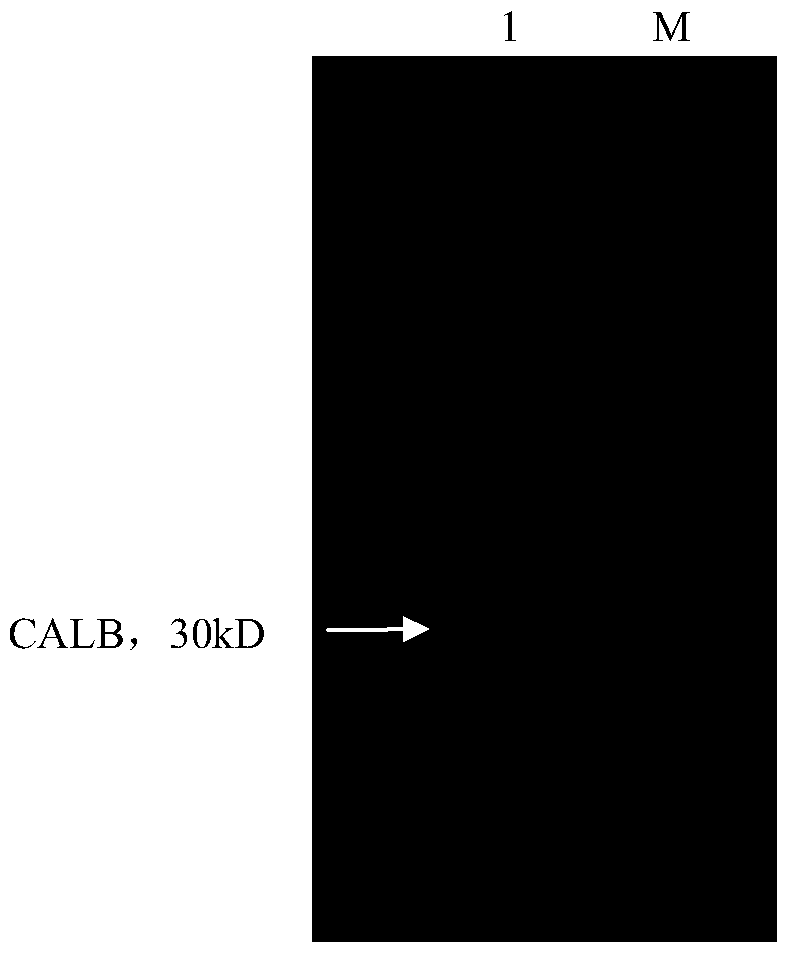
Lipase calb mutant, its preparation method and application
A mutant and lipase technology, applied in the field of genetic engineering, can solve the problems of enzyme instability, insufficient enzyme source, substrate water insolubility, etc., and achieve the effect of mass production and high expression activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1 constructs lipase CALB mutant library
[0027] 1. The coding gene calb of CALB is amplified by lipase-producing Klebsiella sp.;
[0028] 2. The calb gene was connected to the expression plasmid pPICZαA, and the restriction sites were EcoRI and XbaI to obtain the recombinant plasmid pPICZαA-calb;
[0029] 3. Using error-prone PCR technology, using the recombinant plasmid pPICZαA-calb as a template, design primers to amplify and obtain the calb mutant gene;
[0030] 4. The error-prone PCR product obtained in step 3 and the expression plasmid pPICZαA were digested with EcoRI and XbaI and ligated, and the ligated product was transferred into Pichia pastoris X33 competent cells, and coated on bleomycin-resistant (Zec + ) on the YPD plate to obtain the constructed recombinant calb gene mutation library;
[0031] 5. The colonies grown on the YPD plate were transferred to methanol-containing tributyrin (Zec + ) plate, after transferring the plate, place the plat...
Embodiment 2
[0032] The acquisition of embodiment 2 lipase CALB mutant gene
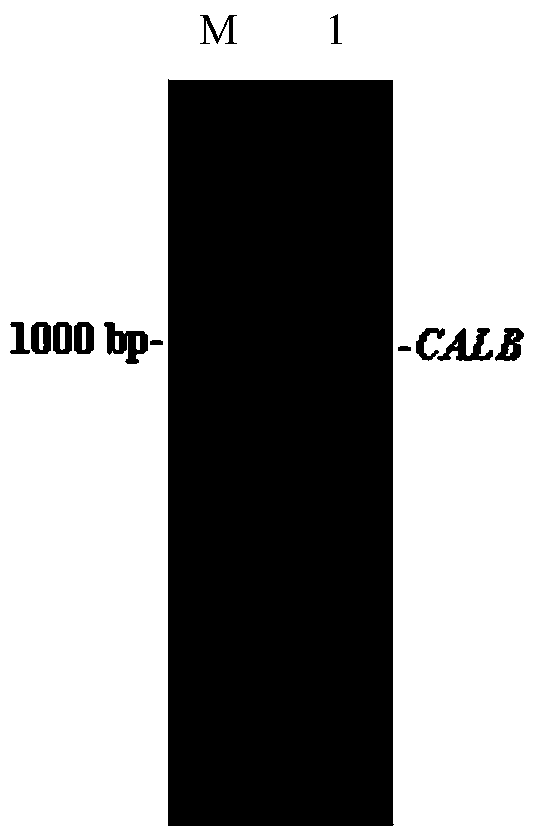
[0033] Using 1 μg of the high lipase-producing strain genomic DNA of Example 1 as a PCR reaction template, the forward primer calb-F: 5′-AAAAAGAATTCAACAAACACGTCGCTGCTATGCTGACGATGCTTATTA-3′, the reverse primer calb-R: 5′-AAAAATCTAGAGTGGTGGTGGTGGTGGTGTCTTTGAGATTTTGGTCTAAAAAA-3′ were designed, wherein The parts in italics are the enzyme cutting sites EcoRI and XbaI, respectively. In order to ensure the correct reading frame, an additional five bases were added to the 5' ends of the forward and reverse primers. The PCR reaction was carried out in a total volume of 50 μL, and the reaction conditions were as follows: denaturation at 94°C for 5 minutes followed by cycling at 94°C for 50 s, annealing at 58°C for 1 minute, extension at 72°C for 2 minutes, a total of 30 cycles, and extension at 72°C for 10 minutes. Take 3 μL of PCR amplification products for agarose gel electrophoresis verification, the results are as foll...
Embodiment 3
[0034] The construction of embodiment 3 expression vector pET-28b (+)-calb
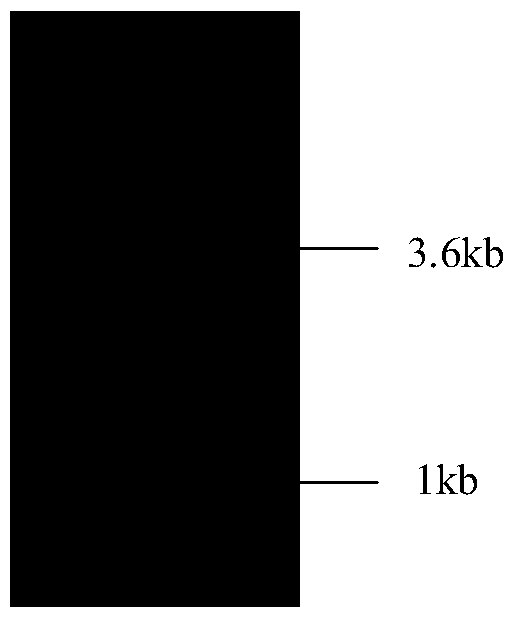
[0035] Gel recovery The PCR product of Example 2 and the pPICZαA vector were double-digested with EcoRI and XbaI respectively, and recovered by a gel recovery kit, then ligated (16°C, 16h), transformed into DH5α competent cells, and positive clones were selected. After extracting the plasmid, it was verified by enzyme digestion analysis, and the results were as follows: figure 2shown, and carried out DNA sequencing identification, the nucleotide sequence encoding the lipase CALB mutant is shown in SEQ ID No.2, and the amino acid sequence of the CALB mutant is shown in SEQ ID No.1. The constructed expression plasmid was called pPICZαA-calb.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com