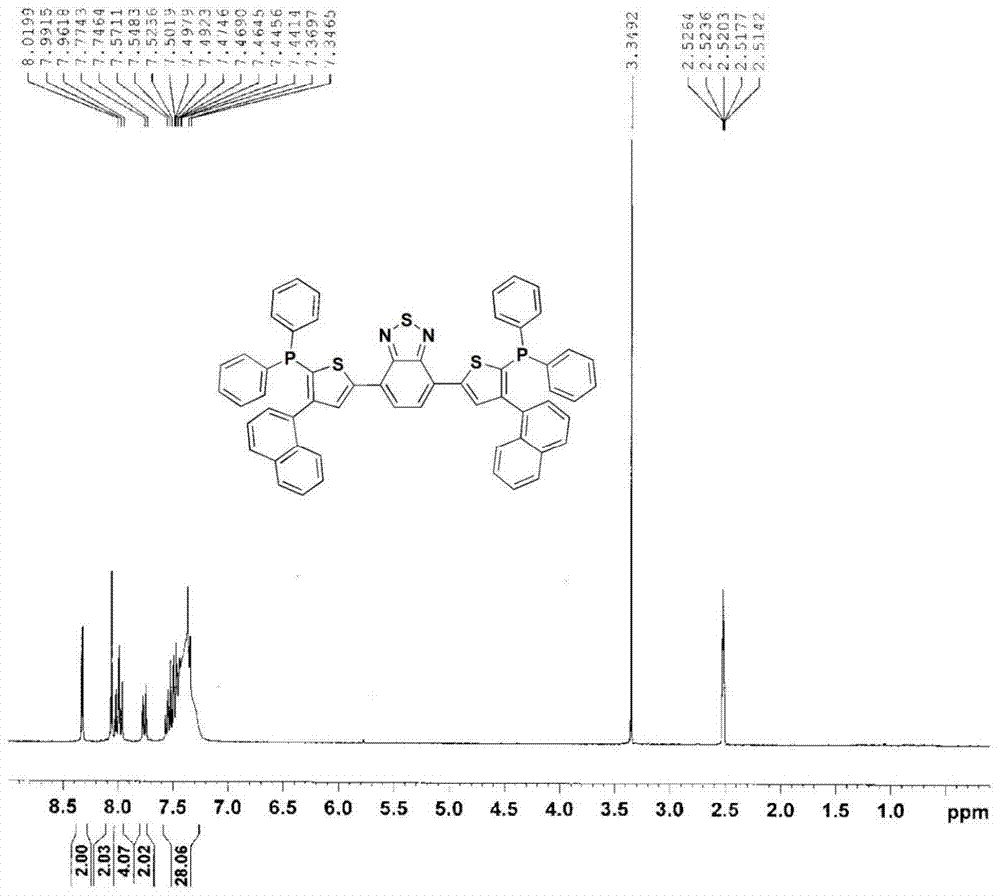
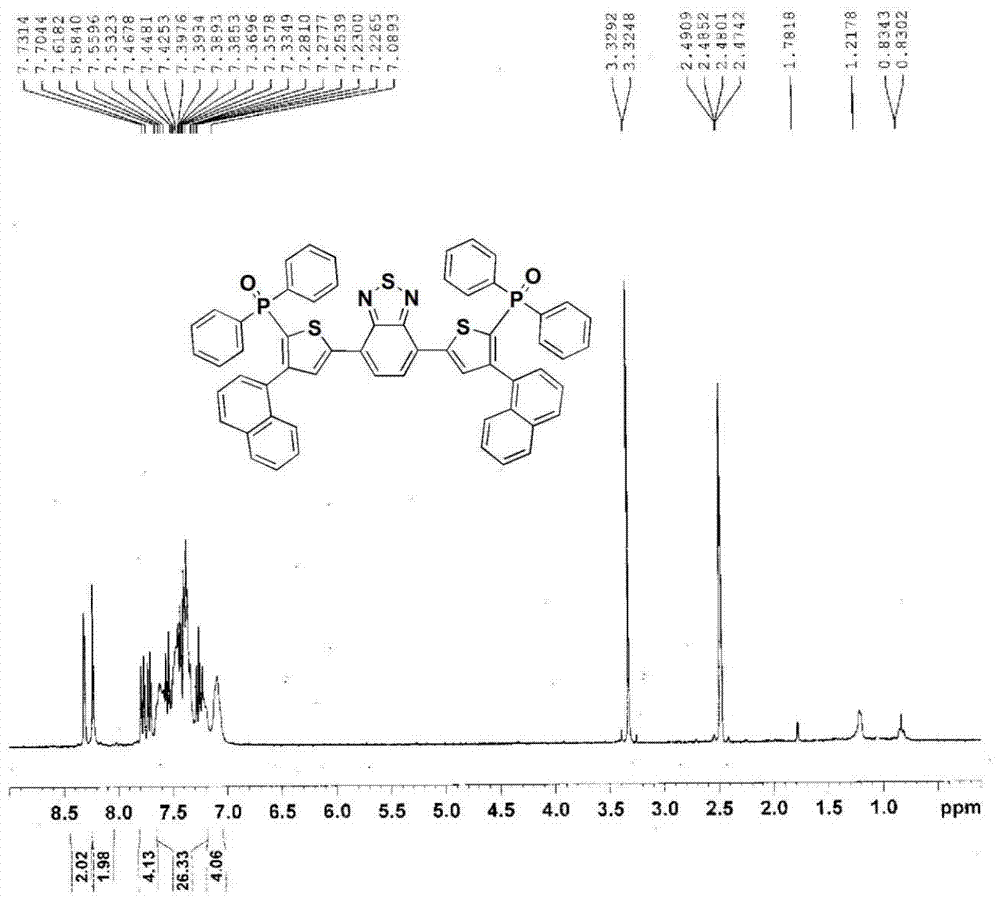
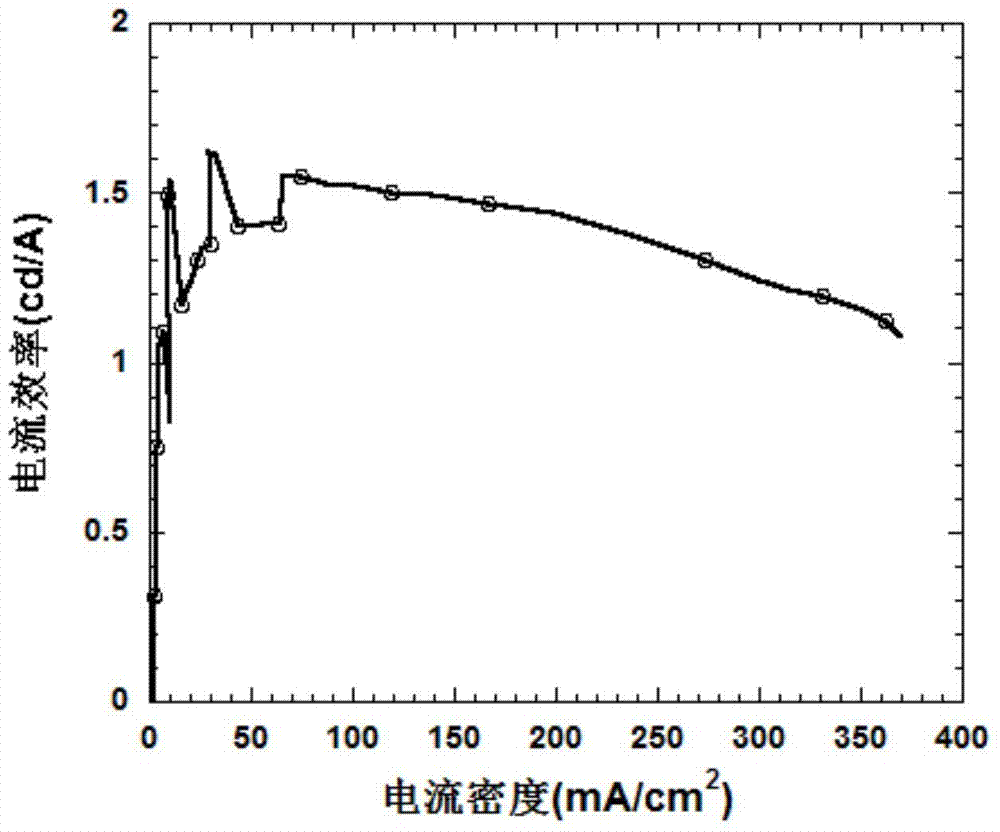
A kind of benzothiadiazole luminescent material containing diphenylphosphine oxide and its preparation and application
A technology of benzothiadiazole and diphenylphosphine oxide is applied in the fields of benzothiadiazole luminescent materials and preparation and application, which can solve problems such as scarcity of luminescent materials, and achieve high product yield, convenient purification, and high solids. The effect of photoluminescence efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1) Dissolve 3-bromothiophene (10.5g, 63.9mmol), 1-naphthaleneboronic acid (10g, 58.1mmol) in toluene (120mL), Na 2 CO 3 In the mixture of aqueous solution (2mol / L, 58mL) and ethanol (10mL), stir and degas with a syringe needle for 30 minutes, and quickly add tetrakis(triphenylphosphine)palladium (336mg, 0.29mmol), the reaction temperature is 80°C, After heating to reflux for 8 h under the protection of nitrogen, it was cooled to room temperature and extracted with dichloromethane. Anhydrous MgSO for organic layer 4 Dry, filter, remove the solvent under reduced pressure, and then separate with a silica gel column. The eluent is petroleum ether. The crude product is refluxed with ethanol to obtain 3-(1-naphthyl)thiophene as a white solid. The above reaction is shown in the following formula:
[0042]
[0043] 3-(1-naphthyl)thiophene (10g, 47.6mmol) and 3.64g LiClO 4 -SiO 2 (1:4) was added into dichloromethane (80ml), stirred evenly, and then N-bromosuccinimide (N...
Embodiment 2
[0055] (1) Add 1-naphthaleneboronic acid (5.16g, 30mmol), s-tribromobenzene (11.3g, 36mmol), toluene (80mL), ethanol (10mL), and 2M sodium carbonate aqueous solution (30mL) into two-necked flasks, and pass Exhaust by bubbling nitrogen gas for 30 minutes. The catalyst tetrakis(triphenylphosphine)palladium (173mg, 0.15mmol) was quickly added into the reaction flask, and then heated to 90°C for 8 hours under reflux. After the mixture was cooled, it was washed with distilled water and extracted with toluene, and the organic phase was extracted with MgSO 4 After drying, the white solid 1-naphthyl-3,5-dibromobenzene was obtained by column chromatography. The above reaction is shown in the following formula:
[0056]
[0057] 1-Naphthyl-3,5-dibromobenzene (18.1g, 50mmol) was dissolved in 150mL of dry tetrahydrofuran, protected with nitrogen, and cooled to -78°C with liquid nitrogen / isopropanol. Slowly added n-BuLi (2.5M, 20 mL, 50 mmol) dropwise into the reaction flask, the mix...
Embodiment 3
[0065] Step (1) and step (2) are identical with embodiment 2.
[0066] (3) 3-(1-naphthyl)-5-(diphenylphosphoryl)-1-(4,4,5,5-tetramethyl-1,3,2-dioxaboryl -2-yl)benzene (2.26g, 4.4mmol), 4,7-bis(5-bromothiophen-2-yl)-2,1,3-benzothiadiazole (0.916g, 2mmol) was dissolved in toluene (50mL), ethanol (10mL), 2M aqueous sodium carbonate solution (4mL), and exhaust by bubbling nitrogen gas for 30 minutes. The catalyst tetrakis(triphenylphosphine)palladium (69mg, 0.06mmol) was quickly added into the reaction flask, and then heated to 90°C for reflux reaction for 12 hours. Cool to room temperature and extract with toluene. Anhydrous MgSO for organic layer 4 Dry, filter, and separate with a silica gel column after removing the solvent under reduced pressure, and reflux the crude product with n-hexane to obtain the red solid product 4,7-bis(5-(3-(1-naphthyl)-5-(diphenylphosphine) Base) phenylthiophen-2-yl) benzothiadiazole.Above-mentioned reaction is shown in the following formula:
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com