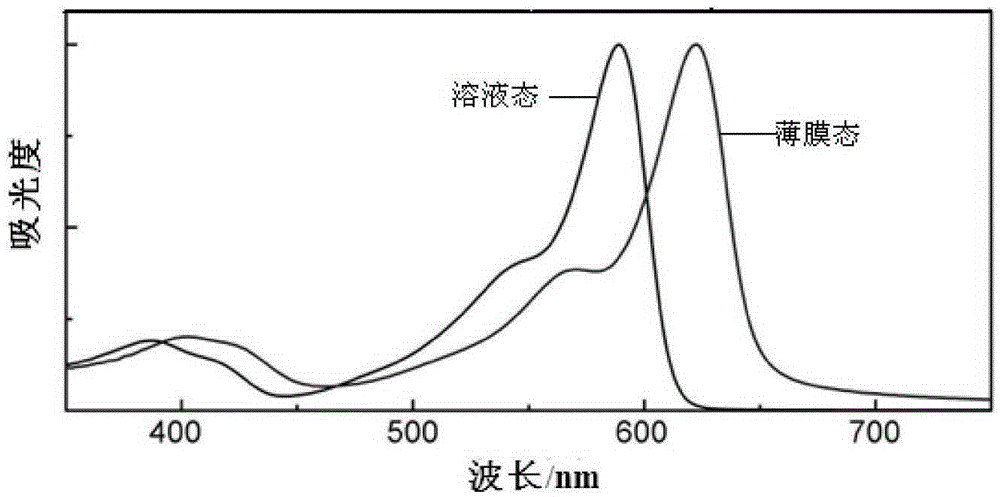
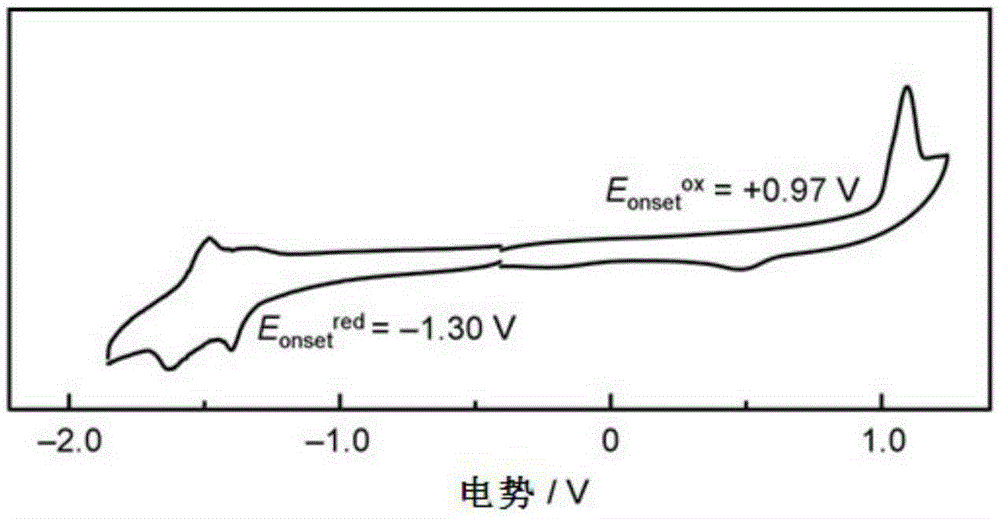
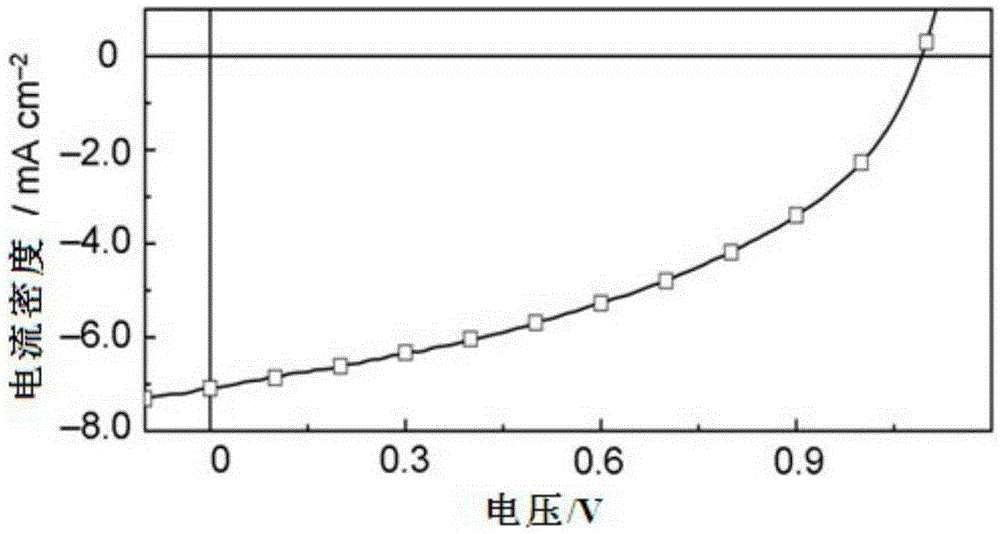
Conjugated polymer based on double boron and nitrogen bridged bipyridine and preparation method and application thereof
A technology of conjugated polymer and bipyridine, which is applied in the field of solar cells, can solve the problems of low energy conversion efficiency of solar cells, and achieve the effects of simple preparation method, high electron mobility, and high photoelectric conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] The preparation method of the above-mentioned BNBP-based conjugated polymer varies according to the structure of -Ar-:
[0047] When the structure of -Ar- is a type structure, the conjugated polymer is prepared by a Still-type reaction. As a preferred solution, the preparation method can be:
[0048] Under the protection of an inert atmosphere (generally using argon), the dibromine monomer, bistrimethyltin monomer, tris (dibenzylidene acetone) dipalladium and tris (o-methyl) phenylphosphine of BNBP are separated by substance The ratio of 1:1:0.02:0.16 is dissolved in toluene solution, the concentration of BNBP's dibromomonomer and bistrimethyltin monomer can be 0.005~0.1M, reflux at 110~120℃ for 24~48h, A still polymerization reaction occurs, and the end-capping agent is added to end-capping. The end-capping agent generally adopts phenylboronic acid and bromobenzene, and after capping, it is purified to obtain a conjugated polymer;
[0049] The reaction formula is as f...
Embodiment 1
[0060] BNBP-BDT polymer, the structural formula is as follows:
[0061]
[0062] The preparation method is: add bisbromo BNBP (155mg, 0.20mmol), bistin salt of benzodithiophene (162mg, 0.21mmol), tris(dibenzylideneacetone) to a clean polymerization bottle after baking Dipalladium (3.7mg, 0.004mmol) and tri(o-methyl)phenylphosphine (9.7mg, 0.032mmol), then vacuumize the system with argon for several times, and add distilled toluene under the condition of avoiding light Solvent (10mL), after reflux at 115°C for 48h, then add phenylboronic acid (100mg, 0.82mmol) and continue to reflux for 3h, then add bromobenzene (200mg, 1.28mmol) and reflux for 3h. The reaction system was cooled to room temperature, dissolved in 100 ml of chloroform, washed with water, dried, and most of the solvent was removed, and the remaining solution was dropped in acetonitrile, and the polymer was precipitated, and the precipitate was sequentially washed with acetone, n-hexane, THF washes away small m...
Embodiment 2
[0067] BNBP-CPDT polymer, the structural formula is as follows:
[0068]
[0069] The preparation method is: add CPDT bistin salt (153.3mg, 0.21mmol), BNBP (154.9mg, 0.20mmol), tris(dibenzylideneacetone) dipalladium (3.7mg , 0.004mmol) and tri(ortho-methyl)phenylphosphine (9.7mg, 0.032mmol), then vacuumize and ventilate the system with argon for several times, add distilled toluene solvent (10mL) under the dark state, After reflux at 115°C for 48h, add phenylboronic acid (100mg, 0.82mmol) to reflux for 3h, then add bromobenzene (200mg, 1.28mmol) to reflux for 3h. The reaction system was cooled to room temperature, dissolved in 100 ml of chloroform, washed with water, dried, and most of the solvent was removed, and the remaining solution was dropped in acetonitrile, and the polymer was precipitated, and the precipitate was sequentially washed with acetone, n-hexane, THF washes away small molecules and catalysts, and finally the polymer is extracted with chloroform. Yield o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical band gap | aaaaa | aaaaa |
| absorption spectrum | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com