Lithium ion battery electrolyte and preparation method thereof, and lithium ion battery
A lithium-ion battery and electrolyte technology, applied in secondary batteries, circuits, electrical components, etc., can solve the problems of battery energy density decrease, inactive material increase, process realization difficulties, etc., to suppress decomposition reaction and improve charge and discharge rate, the effect of fast charge and discharge rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
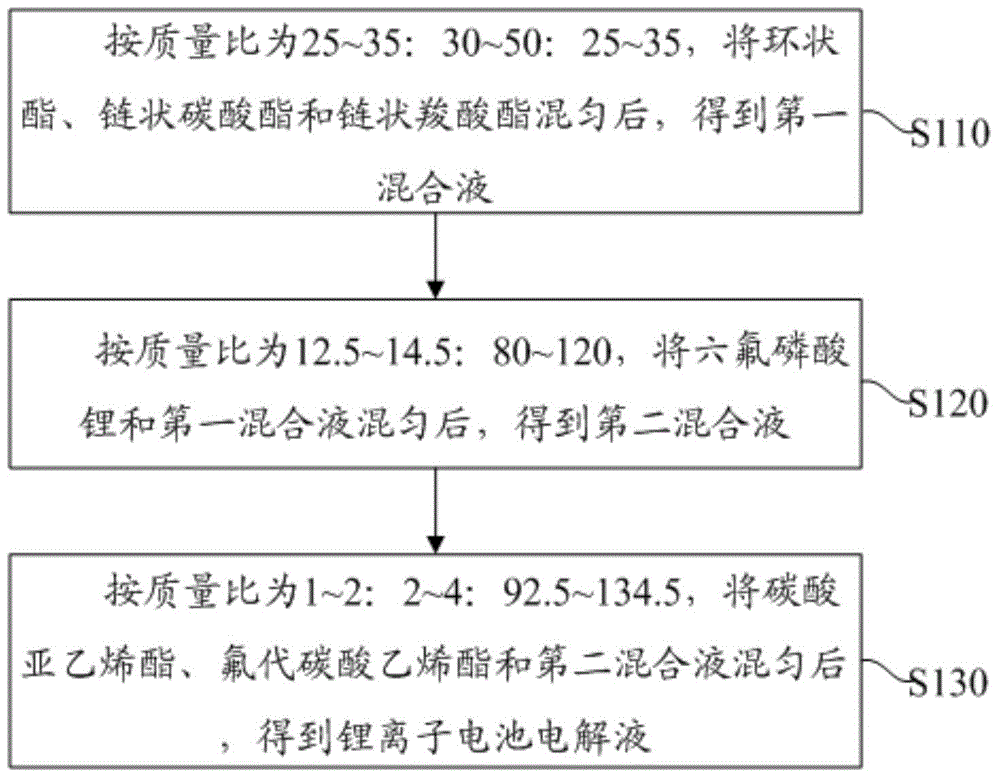
[0032] Such as figure 1 As shown, the preparation method of the above-mentioned lithium-ion battery electrolyte of an embodiment comprises the following steps:
[0033] S110: The mass ratio is 25-35:30-50:25-35, after uniformly mixing the cyclic ester, the chain carbonate and the chain carboxylate to obtain a first mixed solution.
[0034] S120: after the mass ratio is 12.5-14.5:80-120, the lithium hexafluorophosphate and the first mixed liquid are uniformly mixed to obtain the second mixed liquid.
[0035] S130: Mix the vinylene carbonate, the fluoroethylene carbonate and the second mixed solution according to the mass ratio of 1-2:2-4:92.5-134.5 to obtain an electrolyte solution for the lithium-ion battery.
[0036] As another example, after mixing the cyclic ester, chain carbonate and chain carboxylate, let it stand at room temperature for half an hour. In another example, after mixing the lithium hexafluorophosphate and the first mixed solution, they were left to stand a...
Embodiment 1
[0054] In a glove box filled with inert gas argon (moisture content<10PPM, oxygen content<10PPM), 25g ethylene carbonate, 25g propyl acetate, 20g methyl ethyl carbonate, 20g diethyl carbonate and 10g propylene carbonate were used Stir with a magnetic stirrer for 20 minutes, then slowly add 12.5g of lithium hexafluorophosphate, continue to stir with a magnetic stirrer for 20 minutes, finally, add 4g of fluoroethylene carbonate and 1g of vinylene carbonate, and then stir with a magnetic stirrer for 20 minutes to obtain a lithium ion battery electrolyte.
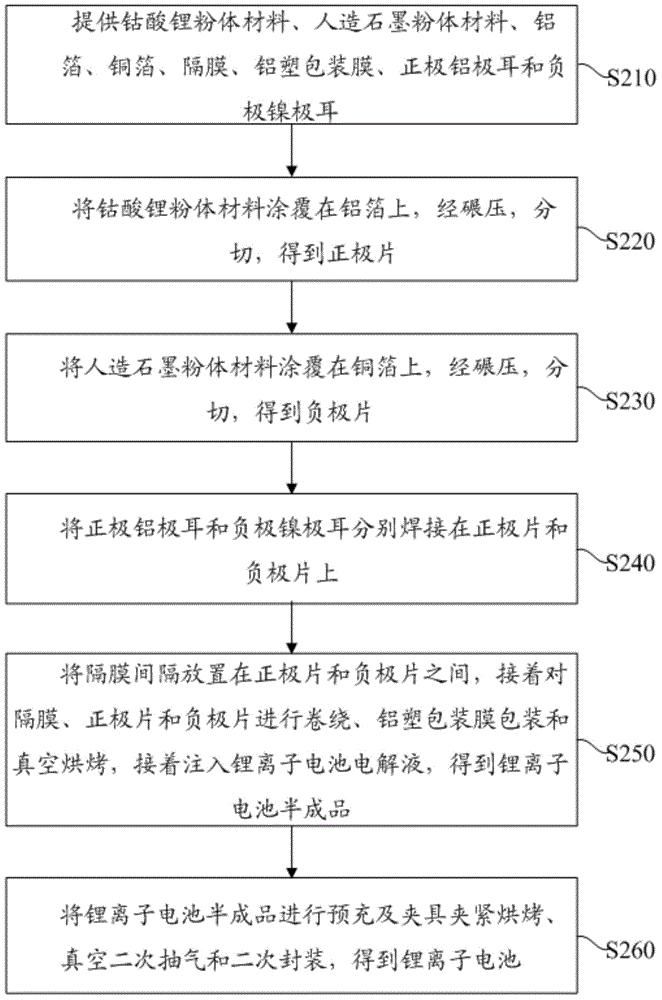
[0055] Provide lithium cobalt oxide powder material with a median particle size of 12 μm, artificial graphite powder material with a median particle size of 18 μm, aluminum foil, copper foil, separator, aluminum-plastic packaging film, positive aluminum tab and negative nickel tab. The lithium cobaltate powder material with a median particle size of 12 μm is coated on an aluminum foil, rolled, and cut to obtain a positive elect...
Embodiment 2
[0057] In a glove box filled with inert gas argon (moisture content<10PPM, oxygen content<10PPM), 20g ethylene carbonate, 20g propyl acetate, 10g ethyl acetate, 40g methyl ethyl carbonate and 10g gamma-butyrolactone were used Stir with a magnetic stirrer for 20 minutes, then slowly add 13.5g of lithium hexafluorophosphate, continue to stir with a magnetic stirrer for 20 minutes, finally, add 3g of fluoroethylene carbonate and 1.5g of vinylene carbonate, and then stir with a magnetic stirrer for 20 minutes to obtain lithium ion battery electrolyte.
[0058] Provide lithium cobalt oxide powder material with a median particle size of 13 μm, artificial graphite powder material with a median particle size of 20 μm, aluminum foil, copper foil, separator, aluminum-plastic packaging film, positive aluminum tab and negative nickel tab. The lithium cobalt oxide powder material with a median particle size of 13 μm is coated on an aluminum foil, rolled, and cut to obtain a positive electr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Median particle size | aaaaa | aaaaa |
| Median particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com