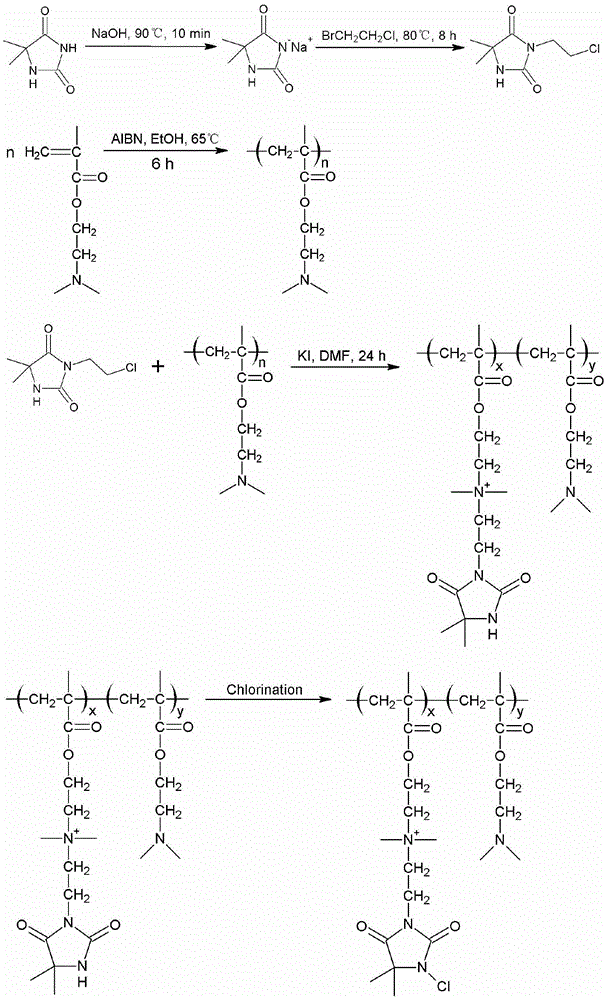Annular halamine type polymeric antibacterial agent containing quaternary ammonium group and preparation method and application of polymeric antibacterial agent
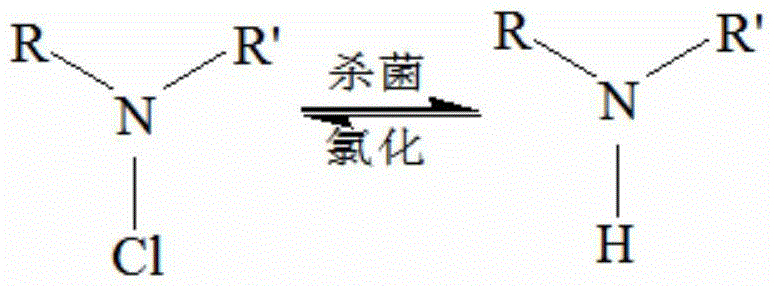
A technology of polymer antibacterial agent and cyclic halamine, which is applied in the synthesis of polymer antibacterial agent and antibacterial field, can solve the problems of high price of synthetic raw materials, less application range, easy penetration into the skin, etc., and achieves good cell compatibility. , excellent antibacterial properties, high antibacterial efficiency effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
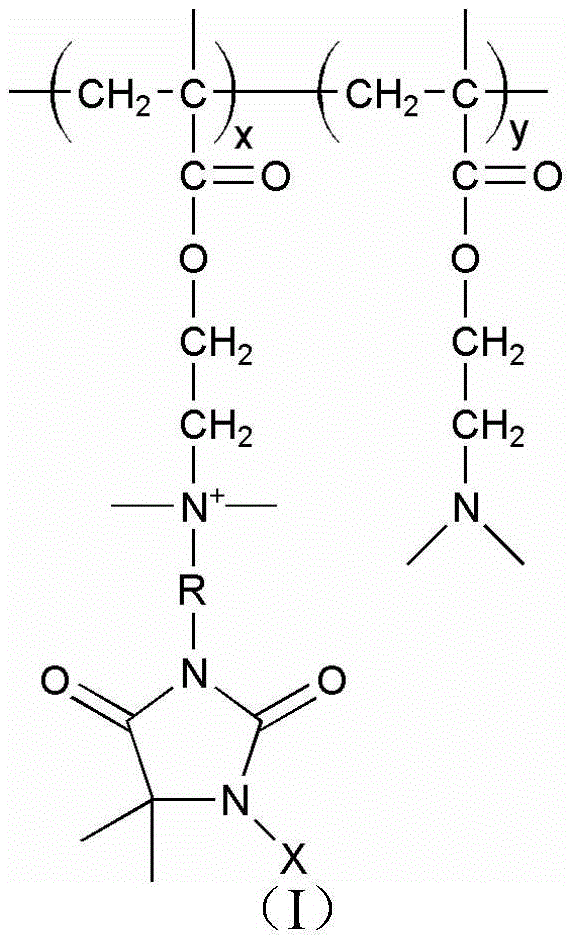
[0058] (1) Synthesis of cyclic small molecule haloamine antibacterial agent precursor: Weigh 12.9g (0.1mol) of 5,5-dimethylhydantoin in a 250mL round bottom flask, add 100mL of ethanol to dissolve it, and then add 4g (0.1mol) sodium hydroxide, put the round bottom flask in an oil bath environment and keep stirring, heat up to 100°C for 10 minutes, then cool down to 75°C, slowly add 14.34g (0.1mol) 1-bromo-2-chloroethyl Alkanes, condensed back to 10h. After the reaction is completed, the solvent is removed by rotary evaporation, the mixture of ethyl acetate and water is separated, and the ethyl acetate part is taken, purified and dried by rotary evaporation to obtain white crystals, which are ring-shaped small molecule haloamine antibacterial agents Precursor 3-(2'-chloroethyl)-5,5-dimethylhydantoin.
[0059] (2) Synthesis of high molecular self-polymer: Weigh 8g of dimethylaminoethyl methacrylate in a 100mL three-neck flask, add 20mL of ethanol solution, then add 0.08g of azo...
Embodiment 2
[0063] (1) Synthesis of cyclic small molecule haloamine antibacterial agent precursor: Weigh 12.9g (0.1mol) of 5,5-dimethylhydantoin in a 250mL round bottom flask, add 100mL of ethanol to dissolve it, and then add 4g (0.1mol) sodium hydroxide, put the round-bottomed flask in an oil bath environment and keep stirring, heat up to 80°C and react for 30min, slowly add 14.34g (0.1mol) 1-bromo-2-chloroethane dropwise, condense and reflux 8h. After the reaction is completed, the solvent is removed by rotary evaporation, the mixture of ethyl acetate and water is separated, and the ethyl acetate part is taken, purified and dried by rotary evaporation to obtain white crystals, which are ring-shaped small molecule haloamine antibacterial agents Precursor 3-(2'-chloroethyl)-5,5-dimethylhydantoin.
[0064] (2) Synthesis of high molecular self-polymer: Weigh 10g of dimethylaminoethyl methacrylate in a 100mL three-neck flask, add 15mL of ethanol solution, then add 0.1g of azobisisobutylcyan...
Embodiment 3
[0068] (1) Synthesis of cyclic small molecule haloamine antibacterial agent precursor: Weigh 12.9g (0.1mol) of 5,5-dimethylhydantoin in a 250mL round bottom flask, add 100mL of ethanol to dissolve it, and then add 4g (0.1mol) sodium hydroxide, put the round bottom flask in an oil bath environment and keep stirring, heat up to 90°C and react for 10min, then cool down to 80°C, slowly add 14.34g (0.1mol) 1-bromo-2-chloroethyl Alkanes, condensed and refluxed for 8h. After the reaction is completed, the solvent is removed by rotary evaporation, the mixture of ethyl acetate and water is separated, and the ethyl acetate part is taken, purified and dried by rotary evaporation to obtain white crystals, which are ring-shaped small molecule haloamine antibacterial agents Precursor 3-(2'-chloroethyl)-5,5-dimethylhydantoin.
[0069] (2) Synthesis of high molecular self-polymer: Weigh 8g of dimethylaminoethyl methacrylate in a 100mL three-neck flask, add 20mL of ethanol solution, then add ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com