Scylla paramamosain anti-bacterial polypeptide Sp-NPFin and its application
A technology of antibacterial peptides and pseudo-cave crabs, applied in the fields of application, antibacterial drugs, peptides, etc., can solve the problems of less research and achieve the effect of broad antibacterial spectrum, significant antibacterial effect, and fast sterilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 Preparation of the antibacterial polypeptide Sp-NPFin of pseudocave crab
[0036] The amino acid sequence of the antibacterial polypeptide Sp-NPFin of the pseudo-scyllas is:
[0037] Arg-Pro-Ser-Thr-Arg-Ser-Ala-Pro-Gly-Pro-Ala-Ser-His-Ile-Gln-Ala-Leu-Glu-Lys-Thr-Leu-Lys-Phe-Leu-Gln- Leu-Gln-Glu-Leu-Gly-Lys-Ile-Tyr-Ser-His-Met-Thr-Arg-Pro-Arg-Phe-Gly-Lys-Arg-Ser (SEQ ID NO: 01)
[0038] The antimicrobial peptide Sp-NPFin of Scylla sclerophyllus can be obtained with a purity of more than 95% by using the existing solid-phase chemical synthesis method. The antimicrobial polypeptide Sp-NPFin of Scylla pseudocavena in this example was synthesized by Jill Biochemical (Shanghai) Co., Ltd. by solid-phase synthesis method, and the molecular weight of the polypeptide, HPLC and other detection information were provided.
[0039] The physical and chemical parameters of the antibacterial peptide Sp-NPFin are shown in Table 1.
[0040] Table 1 Physicochemical paramete...
Embodiment 2
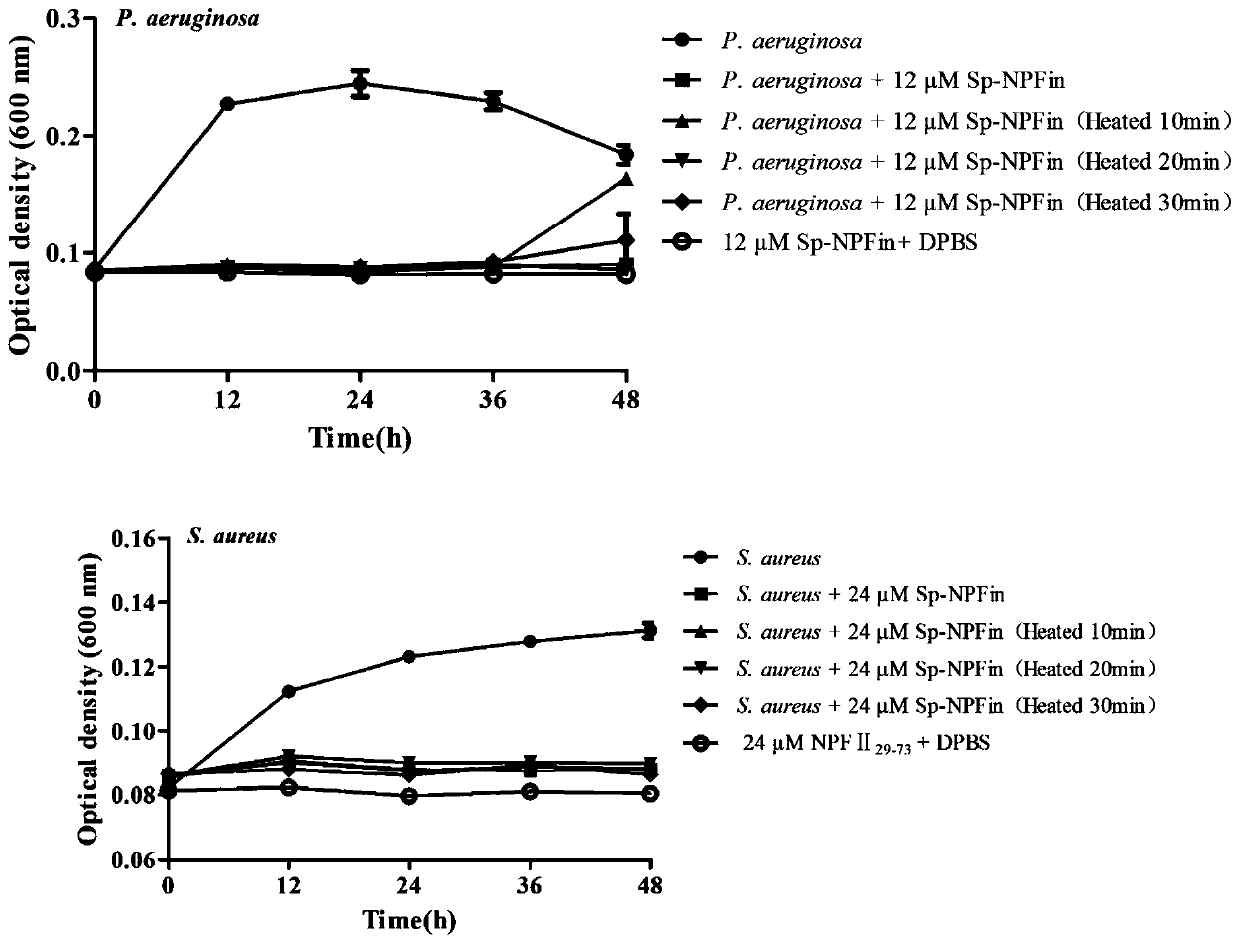
[0043] Example 2 Determination of the minimum inhibitory concentration (MIC: Minimum InhibitionConcentration) and the minimum bactericidal concentration (MBC: Minimum Bactericidal Concentration) of the antibacterial polypeptide Sp-NPFin of the crab crab
[0044] The strains involved in this example are: Staphylococcus aureus, Bacillus subtilis, Corynebacterium glutamicum, Bacillus cereus, Staphylococcus epidermidis (Staphylococcus epidermidis), Micrococcus lysodeikticus, Escherichia coli, Pseudomonas Aeruginosa, Pseudomonas stutzeri, Shigella flexneri Shigella flexneri, Pseudomonas fluorescens, Aeromonas hydrophila, Vibrio harveyi, Vibrio fluvialis, Vibrio alginolyticus (Vibrio alginolyticus), Vibrio parahaemolyticus, Candida albicans, Cryptococcus neoformans, Pichia pastoris, Fusarium graminearum, Fusarium oxysporum Fusarium oxysporum, Aspergillus fumigatus, Aspergillus ochraceus, Aspergillus niger, Fusarium solani. Pichia pastoris GS115 was purchased from Invitrogen, and oth...
Embodiment 3
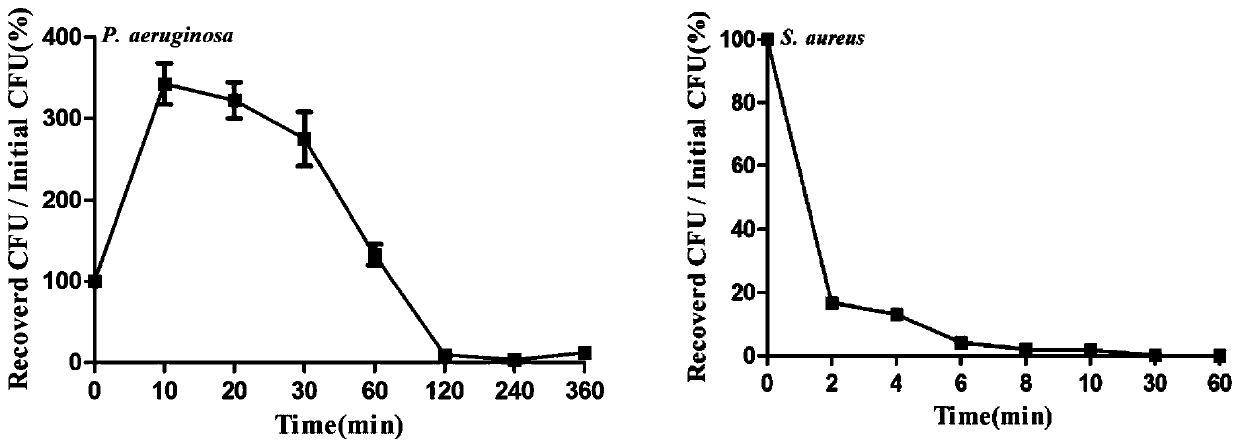
[0060] Example 3 Scylla pseudocaveus antibacterial polypeptide Sp-NPFin bactericidal kinetic curve
[0061] Pseudomonas aeruginosa and Staphylococcus aureus were selected as the test bacteria, and the bactericidal kinetics of the antibacterial polypeptide Sp-NPFin of Scylla syringae were determined.
[0062] The specific method is similar to the antibacterial activity assay described in Example 2. Adjust the final concentration of Sp-NPFin to 1 times MBC (Pseudomonas aeruginosa: 12 μM, Staphylococcus aureus: 24 μM). At 10min, 30min, 60min, 120min, 180min, 360min after co-incubation, mix the blank control group, negative control group, and test group in the 96-well cell culture plate, draw 6 μL of Pseudomonas aeruginosa suspension and dilute to 600 μL In DPBS, after mixing, pipette 40 μL and spread it on a nutrient broth plate, and incubate it upside down at 37°C for 1-2 days, record the number of single clones of Pseudomonas aeruginosa, and calculate the bactericidal index. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com