A kind of modified mesoporous silica adsorbent and its preparation method and application
A mesoporous silica and adsorbent technology, applied in chemical instruments and methods, silicon compounds, inorganic chemistry, etc., to achieve the effects of convenient operation, simple preparation process, and high grafting rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: A preparation method of modified mesoporous silica adsorbent
[0030] Dissolve 3.5g of 1,4,7,10-tetraazacyclododecane (Cyclen), 4g of potassium iodide, and 10g of potassium carbonate in 150mL of anhydrous tetrahydrofuran. Porous silica (precursor chlorine-based functionalized mesoporous silica (SBA-15-Cl) preparation, refer to the relevant literature C.Yu, et al.Advanced Materials.14 (23) (2002) 1742), Reflux reaction under continuous stirring for 48 hours, after cooling to room temperature, the product was separated by suction filtration, washed alternately with ethanol and water several times, and vacuum-dried at 75°C for 12 hours to obtain a modified mesoporous silica adsorbent.
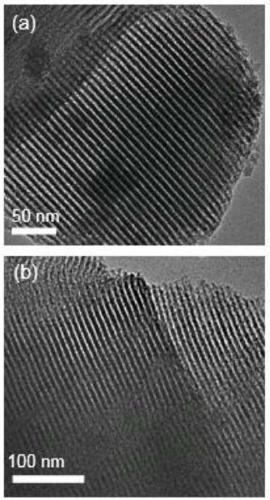
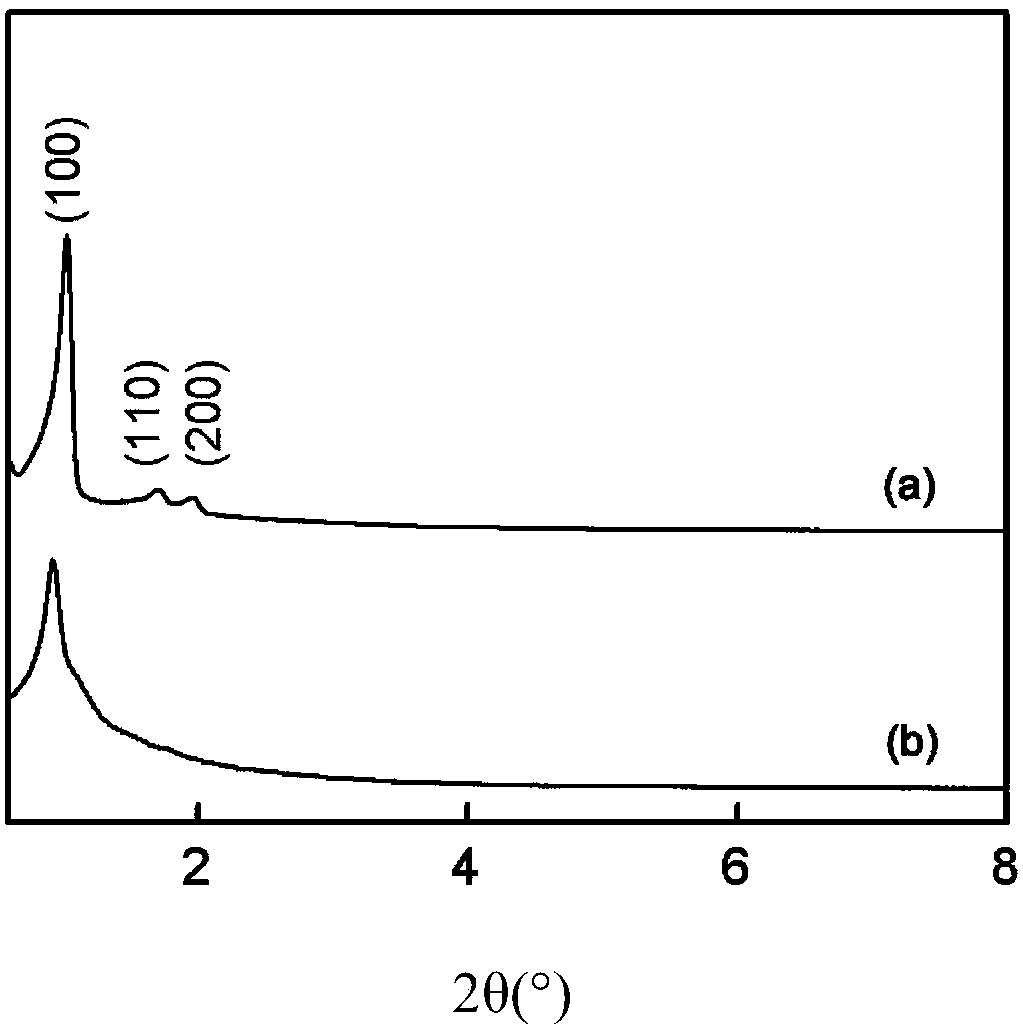
[0031] figure 1 (a) is the transmission electron microscope photo of mesoporous silica (SBA-15-Cl) functionalized with precursor chlorine groups, and the ordered pore structure can be clearly observed, and the pore diameter is about 10nm, which belongs to the mesoporous range; ...
Embodiment 2
[0033] Embodiment 2: the modified mesoporous silica adsorbent obtained in embodiment 1 is to the recovery of palladium in nitric acid medium
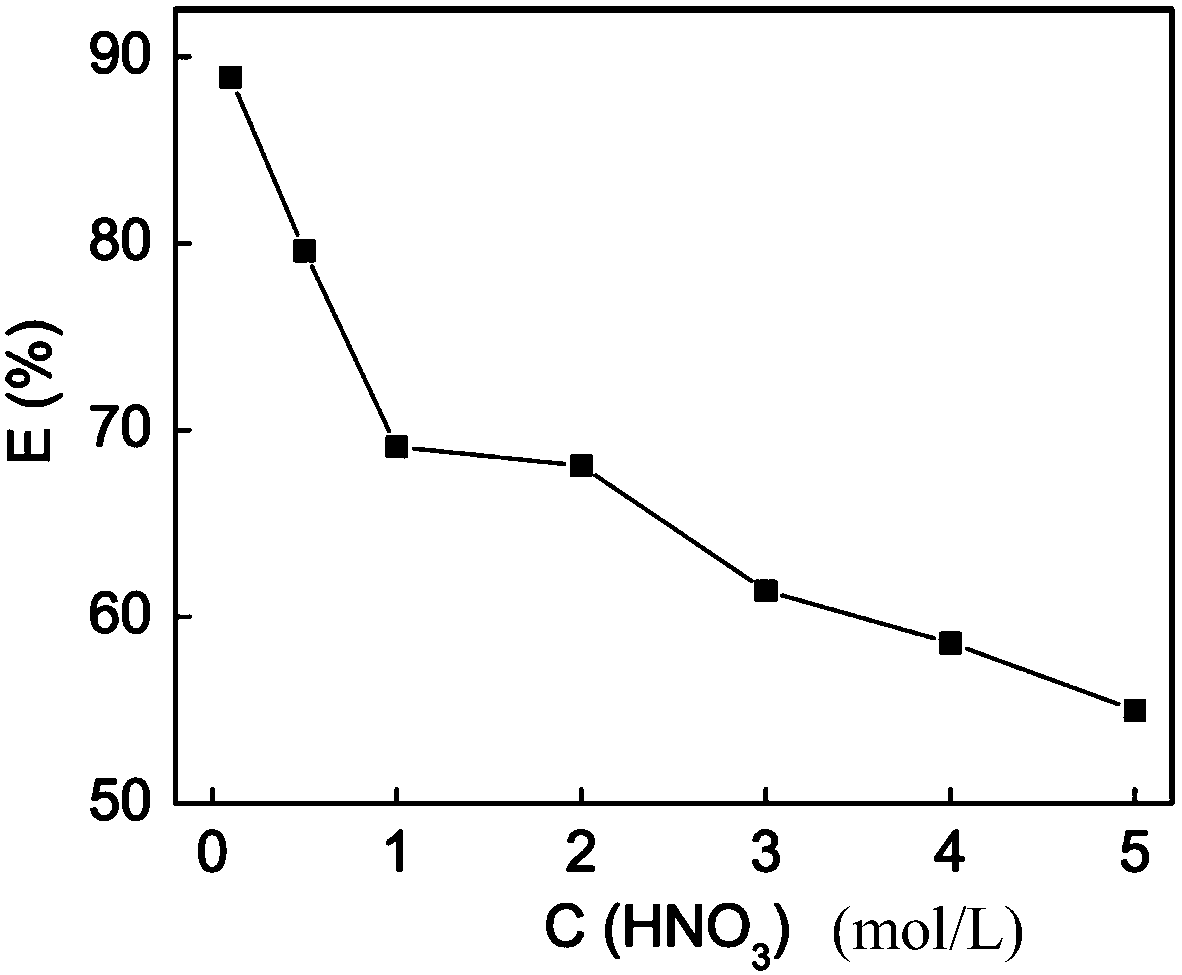
[0034]Pipette 20 mL of a 50 mg / L palladium solution with a nitric acid acidity of 0.1 mol / L in a conical flask, add 0.02 g of the modified mesoporous silica adsorbent obtained in Example 1, and after ultrasonication for 5 min, the Shake at 200r / min for 24h in a constant temperature air bath. After centrifuging the adsorbent, the remaining clear liquid was taken out, and the mass concentration of palladium was measured by atomic absorption spectroscopy, and the adsorption rate E=89.1% of palladium was calculated by the adsorbent according to the adsorption equilibrium formula.
Embodiment 3
[0035] Embodiment 3: the modified mesoporous silica adsorbent obtained in embodiment 1 is to the recovery of palladium in nitric acid medium
[0036] Pipette 20 mL of a 50 mg / L palladium solution with a nitric acid acidity of 0.5 mol / L in an Erlenmeyer flask, add 0.02 g of the modified mesoporous silica adsorbent obtained in Example 1, and after ultrasonication for 5 min, at 25 ± 0.2 ° C Shake at 200r / min for 24h in a constant temperature air bath. After centrifuging the adsorbent, the remaining clear liquid was taken out, and the mass concentration of palladium was measured by atomic absorption spectrometry, and the adsorption rate E=80.6% of the adsorbent to palladium was calculated according to the adsorption equilibrium formula.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com